Dynamics of the Ligand Binding Domain Layer during AMPA Receptor Activation
- PMID: 26910426
- PMCID: PMC4776031
- DOI: 10.1016/j.bpj.2015.12.033
Dynamics of the Ligand Binding Domain Layer during AMPA Receptor Activation
Abstract
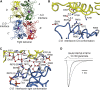
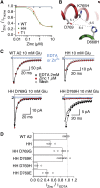
Ionotropic glutamate receptors are postsynaptic tetrameric ligand-gated channels whose activity mediates fast excitatory transmission. Glutamate binding to clamshell-shaped ligand binding domains (LBDs) triggers opening of the integral ion channel, but how the four LBDs orchestrate receptor activation is unknown. Here, we present a high-resolution x-ray crystal structure displaying two tetrameric LBD arrangements fully bound to glutamate. Using a series of engineered metal ion trapping mutants, we showed that the more compact of the two assemblies corresponds to an arrangement populated during activation of full-length receptors. State-dependent cross-linking of the mutants identified zinc bridges between the canonical active LBD dimers that formed when the tetramer was either fully or partially bound by glutamate. These bridges also stabilized the resting state, consistent with the recently published full-length apo structure. Our results provide insight into the activation mechanism of glutamate receptors and the complex conformational space that the LBD layer can sample.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

