Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes
- PMID: 26910618
- PMCID: PMC4854920
- DOI: 10.1111/acel.12456
Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes
Erratum in
-
Corrigendum.Aging Cell. 2017 Aug;16(4):900. doi: 10.1111/acel.12609. Aging Cell. 2017. PMID: 28699313 Free PMC article. No abstract available.
Abstract
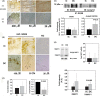
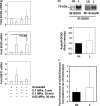
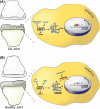
Changes in the content of aggrecan, an essential proteoglycan of articular cartilage, have been implicated in the pathophysiology of osteoarthritis (OA), a prevalent age-related, degenerative joint disease. Here, we examined the effect of SOX9 acetylation on ACAN transactivation in the context of osteoarthritis. Primary chondrocytes freshly isolated from degenerated OA cartilage displayed lower levels of ACAN mRNA and higher levels of acetylated SOX9 compared with cells from intact regions of OA cartilage. Degenerated OA cartilage presented chondrocyte clusters bearing diffused immunostaining for SOX9 compared with intact cartilage regions. Primary human chondrocytes freshly isolated from OA knee joints were cultured in monolayer or in three-dimensional alginate microbeads (3D). SOX9 was hypo-acetylated in 3D cultures and displayed enhanced binding to a -10 kb ACAN enhancer, a result consistent with higher ACAN mRNA levels than in monolayer cultures. It also co-immunoprecipitated with SIRT1, a major deacetylase responsible for SOX9 deacetylation. Finally, immunofluorescence assays revealed increased nuclear localization of SOX9 in primary chondrocytes treated with the NAD SIRT1 cofactor, than in cells treated with a SIRT1 inhibitor. Inhibition of importin β by importazole maintained SOX9 in the cytoplasm, even in the presence of NAD. Based on these data, we conclude that deacetylation promotes SOX9 nuclear translocation and hence its ability to activate ACAN.
Keywords: Aging; SIRT1; SOX9; acetylation; aggrecan; cartilage; nucleus; osteoarthritis.
© 2016 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
-
- Baltus GA, Kowalski MP, Zhai H, Tutter AV, Quinn D, Wall D, Kadam S (2009) Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells 27, 2175–2184. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

