Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier
- PMID: 26910903
- PMCID: PMC4766093
- DOI: 10.1371/journal.pone.0148613
Hyperthermic Laser Ablation of Recurrent Glioblastoma Leads to Temporary Disruption of the Peritumoral Blood Brain Barrier
Abstract
Background: Poor central nervous system penetration of cytotoxic drugs due to the blood brain barrier (BBB) is a major limiting factor in the treatment of brain tumors. Most recurrent glioblastomas (GBM) occur within the peritumoral region. In this study, we describe a hyperthemic method to induce temporary disruption of the peritumoral BBB that can potentially be used to enhance drug delivery.
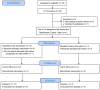
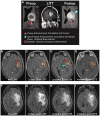
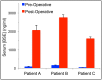
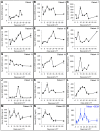
Methods: Twenty patients with probable recurrent GBM were enrolled in this study. Fourteen patients were evaluable. MRI-guided laser interstitial thermal therapy was applied to achieve both tumor cytoreduction and disruption of the peritumoral BBB. To determine the degree and timing of peritumoral BBB disruption, dynamic contrast-enhancement brain MRI was used to calculate the vascular transfer constant (Ktrans) in the peritumoral region as direct measures of BBB permeability before and after laser ablation. Serum levels of brain-specific enolase, also known as neuron-specific enolase, were also measured and used as an independent quantification of BBB disruption.
Results: In all 14 evaluable patients, Ktrans levels peaked immediately post laser ablation, followed by a gradual decline over the following 4 weeks. Serum BSE concentrations increased shortly after laser ablation and peaked in 1-3 weeks before decreasing to baseline by 6 weeks.
Conclusions: The data from our pilot research support that disruption of the peritumoral BBB was induced by hyperthemia with the peak of high permeability occurring within 1-2 weeks after laser ablation and resolving by 4-6 weeks. This provides a therapeutic window of opportunity during which delivery of BBB-impermeant therapeutic agents may be enhanced.
Trial registration: ClinicalTrials.gov NCT01851733.
Conflict of interest statement
Figures

Comment in
-
Laser treatment opens temporary window in blood-brain barrier.BMJ. 2016 Feb 24;352:i1154. doi: 10.1136/bmj.i1154. BMJ. 2016. PMID: 26917783 No abstract available.
References
-
- Parkin DM. Global cancer statistics in the year 2000. The Lancet Oncology. 2001;2(9):533–43. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–66. 10.1016/S1470-2045(09)70025-7 - DOI - PubMed
-
- Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–11. Epub 1980/09/01. . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

