Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X
- PMID: 26911483
- PMCID: PMC4765185
- DOI: 10.1186/s12936-016-1161-5
Plasmodium falciparum ookinete expression of plasmepsin VII and plasmepsin X
Abstract
Background: Plasmodium invasion of the mosquito midgut is a population bottleneck in the parasite lifecycle. Interference with molecular mechanisms by which the ookinete invades the mosquito midgut is one potential approach to developing malaria transmission-blocking strategies. Plasmodium aspartic proteases are one such class of potential targets: plasmepsin IV (known to be present in the asexual stage food vacuole) was previously shown to be involved in Plasmodium gallinaceum infection of the mosquito midgut, and plasmepsins VII and plasmepsin X (not known to be present in the asexual stage food vacuole) are upregulated in Plasmodium falciparum mosquito stages. These (and other) parasite-derived enzymes that play essential roles during ookinete midgut invasion are prime candidates for transmission-blocking vaccines.
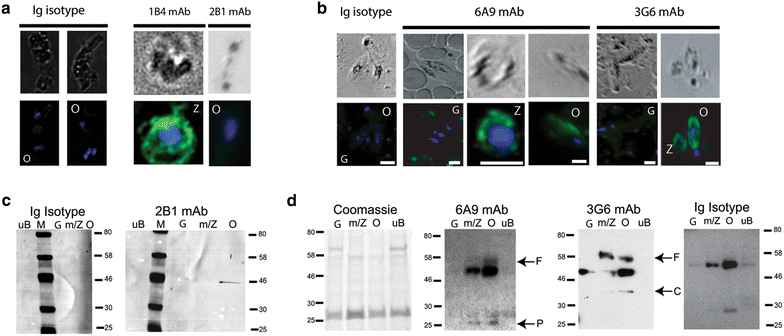
Methods: Reverse transcriptase PCR (RT-PCR) was used to determine timing of P. falciparum plasmepsin VII (PfPM VII) and plasmepsin X (PfPM X) mRNA transcripts in parasite mosquito midgut stages. Protein expression was confirmed by western immunoblot and immunofluorescence assays (IFA) using anti-peptide monoclonal antibodies (mAbs) against immunogenic regions of PfPM VII and PfPM X. These antibodies were also used in standard membrane feeding assays (SMFA) to determine whether inhibition of these proteases would affect parasite transmission to mosquitoes. The Mann-Whitney U test was used to analyse mosquito transmission assay results.
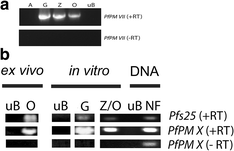
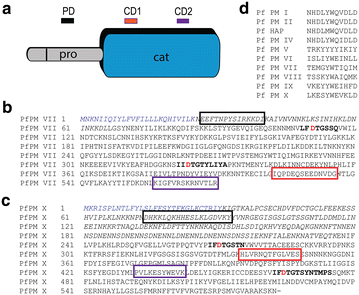
Results: RT-PCR, western immunoblot and immunofluorescence assay confirmed expression of PfPM VII and PfPM X in mosquito stages. Whereas PfPM VII was expressed in zygotes and ookinetes, PfPM X was expressed in gametes, zygotes, and ookinetes. Antibodies against PfPM VII and PfPM X decreased P. falciparum invasion of the mosquito midgut when used at high concentrations, indicating that these proteases play a role in Plasmodium mosquito midgut invasion. Failure to generate genetic knockouts of these genes limited determination of the precise role of these proteases in parasite transmission but suggests that they are essential during the intraerythrocytic life cycle.
Conclusions: PfPM VII and PfPM X are present in the mosquito-infective stages of P. falciparum. Standard membrane feeding assays demonstrate that antibodies against these proteins reduce the infectivity of P. falciparum for mosquitoes, suggesting their viability as transmission-blocking vaccine candidates. Further study of the role of these plasmepsins in P. falciparum biology is warranted.
Figures
Similar articles
-
Apical surface expression of aspartic protease Plasmepsin 4, a potential transmission-blocking target of the plasmodium ookinete.J Biol Chem. 2010 Mar 12;285(11):8076-83. doi: 10.1074/jbc.M109.063388. Epub 2010 Jan 7. J Biol Chem. 2010. PMID: 20056606 Free PMC article.
-
Expression and characterisation of plasmepsin I from Plasmodium falciparum.Eur J Biochem. 1997 Mar 1;244(2):552-60. doi: 10.1111/j.1432-1033.1997.00552.x. Eur J Biochem. 1997. PMID: 9119023
-
Differential roles of an Anopheline midgut GPI-anchored protein in mediating Plasmodium falciparum and Plasmodium vivax ookinete invasion.Infect Genet Evol. 2014 Dec;28:635-47. doi: 10.1016/j.meegid.2014.05.025. Epub 2014 Jun 11. Infect Genet Evol. 2014. PMID: 24929123 Free PMC article.
-
Interactions of human malaria parasites, Plasmodium vivax and P.falciparum, with the midgut of Anopheles mosquitoes.Med Vet Entomol. 1997 Jul;11(3):290-6. doi: 10.1111/j.1365-2915.1997.tb00409.x. Med Vet Entomol. 1997. PMID: 9330262 Review.
-
Plasmodium P47: a key gene for malaria transmission by mosquito vectors.Curr Opin Microbiol. 2017 Dec;40:168-174. doi: 10.1016/j.mib.2017.11.029. Epub 2017 Dec 8. Curr Opin Microbiol. 2017. PMID: 29229188 Free PMC article. Review.
Cited by
-
Targeting the Plasmodium falciparum proteome and organelles for potential antimalarial drug candidates.Heliyon. 2022 Aug;8(8):e10390. doi: 10.1016/j.heliyon.2022.e10390. Epub 2022 Aug 24. Heliyon. 2022. PMID: 36033316 Free PMC article. Review.
-
Comparative single-cell transcriptional atlases of Babesia species reveal conserved and species-specific expression profiles.PLoS Biol. 2022 Sep 22;20(9):e3001816. doi: 10.1371/journal.pbio.3001816. eCollection 2022 Sep. PLoS Biol. 2022. PMID: 36137068 Free PMC article.
-
Deciphering the mechanism of potent peptidomimetic inhibitors targeting plasmepsins - biochemical and structural insights.FEBS J. 2018 Aug;285(16):3077-3096. doi: 10.1111/febs.14598. Epub 2018 Jul 7. FEBS J. 2018. PMID: 29943906 Free PMC article.
-
Plasmodium genomics: an approach for learning about and ending human malaria.Parasitol Res. 2019 Jan;118(1):1-27. doi: 10.1007/s00436-018-6127-9. Epub 2018 Nov 6. Parasitol Res. 2019. PMID: 30402656 Review.
-
Molecular interactions between parasite and mosquito during midgut invasion as targets to block malaria transmission.NPJ Vaccines. 2021 Nov 29;6(1):140. doi: 10.1038/s41541-021-00401-9. NPJ Vaccines. 2021. PMID: 34845210 Free PMC article. Review.
References
-
- WHO: World Malaria Report 2015. Geneva, World Health Organization; 2015.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

