SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis
- PMID: 26911584
- PMCID: PMC4769433
- DOI: 10.1136/bmjopen-2015-009417
SGLT-2 receptor inhibitors for treating patients with type 2 diabetes mellitus: a systematic review and network meta-analysis
Abstract
Objective: Because of the lack of head-to-head trials, the aim was to indirectly compare sodium glucose transporter-2 (SGLT-2) inhibitors in the treatment of type 2 diabetes.
Design: Systematic review and network meta-analysis.
Data sources: MEDLINE and EMBASE were searched from January 2005 to January 2015.
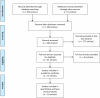
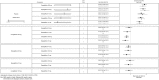
Eligibility criteria: Randomised controlled trials assessing the efficacy of SGLT-2 inhibitors in patients with type 2 diabetes inadequately controlled with diet and exercise alone or metformin monotherapy. Minimum duration 24 weeks. Indirect comparison was undertaken using Bayesian methods.
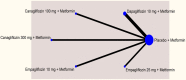
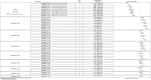
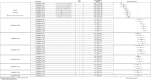
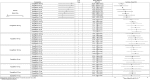
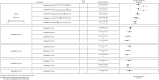
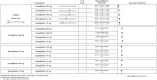
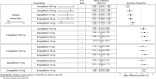
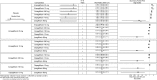
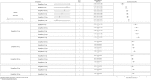
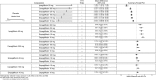
Results: In monotherapy, a greater proportion of patients achieved a glycated haemoglobin (HbA1c) level of <7% on canagliflozin 300 mg than on canagliflozin 100 mg (risk ratio (RR) 0.72%, 95% credible intervals (CrI) 0.59% to 0.87%) and dapagliflozin 10 mg (RR 0.63, 95% CrI 0.48 to 0.85) but there were no significant differences compared with either dose of empagliflozin. In monotherapy, canagliflozin 300 mg reduced HbA1c more than other SGLT-2 inhibitors (mean difference ranged from 0.20% to 0.64%). There were no significant differences in weight reduction. All the flozins reduced systolic blood pressure (SBP) more than placebo, ranging from a reduction of 6 mm Hg with canagliflozin 300-2.6 mm Hg with empagliflozin 10 mg. In dual therapy with metformin, all flozins were more effective than placebo for achieving HbA1c <7%, and reducing HbA1c, weight and SBP. The proportions achieving HbA1c level of <7% were mostly similar. Canagliflozin 300 mg reduced HbA1c more than the other drugs but this just reached statistical significance only against canagliflozin 100 mg (MD 0.15, CrI 0.04 to 0.26).
Conclusions: There were few differences among the SGLT-2 inhibitors, but in monotherapy, the glucose-lowering effect of canagliflozin 300 mg is slightly greater than most other SGLT-2 inhibitors.
Keywords: SGLT2 inhibitors; type 2 diabetes.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures




References
-
- National Institute for Health and Care Excellence. NICE Guidance TA288. Dapagliflozin in combination therapy for treating type 2 diabetes. Secondary NICE Guidance TA288. Dapagliflozin in combination therapy for treating type 2 diabetes 2013. http://www.nice.org.uk/guidance/TA288/chapter/1-guidance
-
- National Institute for Health and Care Excellence. NICE Guidance TA315. Canagliflozin in combination therapy for treating type 2 diabetes. Secondary NICE Guidance TA315. Canagliflozin in combination therapy for treating type 2 diabetes 2014. http://www.nice.org.uk/guidance/TA315/chapter/1-guidance
-
- National Institute for Health and Care Excellence. Empagliflozin combination therapy for treating type 2 diabetes [ID641] GID-TAG441. Secondary Empagliflozin combination therapy for treating type 2 diabetes [ID641] GID-TAG441 2014. http://www.nice.org.uk/guidance/indevelopment/GID-TAG441
-
- Polidori D, Sha S, Mudaliar S et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion results of a randomized, placebo-controlled study. Diabetes Care 2013;36:2154–61. 10.2337/dc12-2391 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
