Conversion to eslicarbazepine acetate monotherapy: A pooled analysis of 2 phase III studies
- PMID: 26911639
- PMCID: PMC4826334
- DOI: 10.1212/WNL.0000000000002497
Conversion to eslicarbazepine acetate monotherapy: A pooled analysis of 2 phase III studies
Abstract
Objective: To assess the efficacy and safety of eslicarbazepine acetate (ESL) monotherapy.
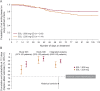
Methods: This post hoc pooled analysis of 2 randomized double-blind studies (093-045 and -046) included adults with partial-onset seizures medically uncontrolled by 1 or 2 antiepileptic drugs (AEDs). Following the baseline period (8 weeks), eligible patients were randomized 2:1 to receive ESL 1,600 mg or 1,200 mg once daily for 18 weeks; the primary endpoint was study exit by meeting predefined exit criteria (signifying worsening seizure control). In each study, treatment was considered effective if the upper 95% confidence limit for exit rate was lower than the historical control threshold (65.3%).
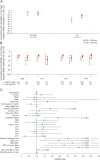
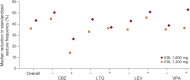
Results: Pooled exit rates were as follows: ESL 1,600 mg = 20.6% (95% confidence interval: 15.6%-26.8%); ESL 1,200 mg = 30.8% (23.0%-40.5%). Use of 2 baseline AEDs or rescue medication, US location, epilepsy duration ≥20 years, and higher maximum baseline seizure frequency were associated with higher exit risks. Median percent reductions in standardized seizure frequency between baseline and the 18-week double-blind period were as follows: ESL 1,600 mg = 43.2%; ESL 1,200 mg = 35.7%; baseline carbamazepine use was associated with smaller reductions. Safety profiles were similar between ESL doses.
Conclusions: Exit rates for ESL monotherapy (1,600 mg and 1,200 mg once daily) were lower than the historical control threshold, irrespective of baseline AED use and region, with no additional safety concerns identified. Clinical factors and location clearly influence treatment responses in conversion-to-monotherapy trials.
Classification of evidence: This pooled analysis provides Class IV evidence that for adults with medically uncontrolled partial-onset seizures, ESL monotherapy is well tolerated and effective.
© 2016 American Academy of Neurology.
Figures
References
-
- Perucca E. Designing clinical trials to assess antiepileptic drugs as monotherapy. CNS Drugs 2008;22:917–938. - PubMed
-
- Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol 2011;10:446–456. - PubMed
-
- Hebeisen S, Pires N, Loureiro AI, et al. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology 2015;89:122–135. - PubMed
-
- Sperling MR, Harvey J, Grinnell T, Cheng H, Blum D; 045 Study Team. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia 2015;56:546–555. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
