Recruitment strategies and challenges in a large intervention trial: Systolic Blood Pressure Intervention Trial
- PMID: 26911833
- PMCID: PMC4965303
- DOI: 10.1177/1740774516631735
Recruitment strategies and challenges in a large intervention trial: Systolic Blood Pressure Intervention Trial
Abstract
Background: The Systolic Blood Pressure Intervention Trial is a multicenter, randomized clinical trial of 9361 participants with hypertension who are ≥50 years old. The trial is designed to evaluate the effect of intensive systolic blood pressure control (systolic blood pressure goal <120 mm Hg) compared to standard control (systolic blood pressure goal <140 mm Hg) on cardiovascular events using commonly prescribed antihypertensive medications and lifestyle modification.
Objective: To describe the recruitment strategies and lessons learned during recruitment of the Systolic Blood Pressure Intervention Trial cohort and five targeted participant subgroups: pre-existing cardiovascular disease, pre-existing chronic kidney disease, age ≥75 years, women, and minorities.
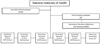
Methods: In collaboration with the National Institutes of Health Project Office and Systolic Blood Pressure Intervention Trial Coordinating Center, five Clinical Center Networks oversaw clinical site selection, recruitment, and trial activities. Recruitment began on 8 November 2010 and ended on 15 March 2013 (about 28 months). Various recruitment strategies were used, including mass mailing, brochures, referrals from healthcare providers or friends, posters, newspaper ads, radio ads, and electronic medical record searches.
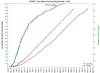
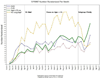
Results: Recruitment was scheduled to last 24 months to enroll a target of 9250 participants; in just over 28 months, the trial enrolled 9361 participants. The trial screened 14,692 volunteers, with 33% of initial screens originating from the use of mass mailing lists. Screening results show that participants also responded to recruitment efforts through referral by Systolic Blood Pressure Intervention Trial staff, healthcare providers, or friends (45%); brochures or posters placed in clinic waiting areas (15%); and television, radio, newspaper, Internet ads, or toll-free numbers (8%). The overall recruitment yield (number randomized/number screened) was 64% (9361 randomized/14,692 screened), 77% for those with cardiovascular disease, 79% for those with chronic kidney disease, 70% for those aged ≥75 years, 55% for women, and 61% for minorities. As recruitment was observed to lag behind expectations, additional clinics were included and inclusion criteria were broadened, keeping event rates and trial power in mind. As overall recruitment improved, a greater focus on subgroup recruitment was implemented.
Conclusion: Systolic Blood Pressure Intervention Trial met its overall projected recruitment goal using diverse, locally adapted enrollment strategies to specifically target persons with cardiovascular disease, chronic kidney disease, ≥75 years old, women, and minority subgroups. The trial exceeded its recruitment goal for minorities but found it a challenge to meet the competing demands of the targeted goals for recruiting into the remaining four subgroups. Important lessons include the imperative to monitor the recruitment process carefully, decide early to add new clinics or modify inclusion and exclusion criteria if recruitment lags, and consider limiting enrollment to subgroups only. We found benefit in using multiple recruitment sources simultaneously; mass mailing produced the largest number of participants, but referrals resulted in the greater randomization yield.
Keywords: Clinical Center Networks; SPRINT; hypertension; recruitment.
© The Author(s) 2016.
Figures
References
-
- Treweek S, Mitchell E, Pitkethly M, et al. Strategies to improve recruitment to randomised controlled trials. Cochrane Database Syst Rev. 2010;4 - PubMed
-
- Lovato LC, Hill K, Hertert S, et al. Recruitment for controlled clinical trials: literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–352. - PubMed
-
- Cosgrove N, Borhani NO, Bailey G, et al. Mass mailing staff experience in a total recruitment program for a clinical trial: the SHEP experience. Systolic Hypertension in the Elderly Program Cooperative Research Group. Control Clin Trials. 1999;20:133–148. - PubMed
-
- SPRINT Protocol. SPRINT Protocol and MOP. 2015
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000445/TR/NCATS NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- UL1 TR000075/TR/NCATS NIH HHS/United States
- UL1 TR001064/TR/NCATS NIH HHS/United States
- UL1 TR000093/TR/NCATS NIH HHS/United States
- UL1 TR000050/TR/NCATS NIH HHS/United States
- UL1 RR025755/RR/NCRR NIH HHS/United States
- UL1 TR001085/TR/NCATS NIH HHS/United States
- UL1 RR024134/RR/NCRR NIH HHS/United States
- UL1 TR000433/TR/NCATS NIH HHS/United States
- UL1 TR001070/TR/NCATS NIH HHS/United States
- HHSN268200900048C/HL/NHLBI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- HHSN268200900040C/HL/NHLBI NIH HHS/United States
- HHSN268200900046C/HL/NHLBI NIH HHS/United States
- UL1 RR025752/RR/NCRR NIH HHS/United States
- UL1 RR025771/RR/NCRR NIH HHS/United States
- HHSN268200900049C/HL/NHLBI NIH HHS/United States
- HHSN268200900047C/HL/NHLBI NIH HHS/United States
- UL1 TR001427/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000439/TR/NCATS NIH HHS/United States
- UL1 TR000073/TR/NCATS NIH HHS/United States
- UL1 TR000002/TR/NCATS NIH HHS/United States
- UL1 TR000105/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous