Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients
- PMID: 26912315
- PMCID: PMC5413532
- DOI: 10.1007/s00134-015-4205-3
Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients
Abstract
Purpose: Danger-associated molecular patterns (DAMPs) released of trauma could contribute to an immune suppressed state that renders patients vulnerable towards nosocomial infections. We investigated DAMP release in trauma patients, starting in the prehospital phase, and assessed its relationship with immune suppression and nosocomial infections.
Methods: Blood was obtained from 166 adult trauma patients at the trauma scene, emergency room (ER), and serially afterwards. Circulating levels of DAMPs and cytokines were determined. Immune suppression was investigated by determination of HLA-DRA gene expression and ex vivo lipopolysaccharide-stimulated cytokine production.
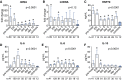
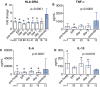
Results: Compared with healthy controls, plasma levels of nuclear DNA (nDNA) and heat shock protein-70 (HSP70) but not mitochondrial DNA were profoundly increased immediately following trauma and remained elevated for 10 days. Plasma cytokines were increased at the ER, and levels of anti-inflammatory IL-10 but not of pro-inflammatory cytokines peaked at this early time-point. HLA-DRA expression was attenuated directly after trauma and did not recover during the follow-up period. Plasma nDNA (r = -0.24, p = 0.006) and HSP70 (r = -0.38, p < 0.0001) levels correlated negatively with HLA-DRA expression. Ex vivo cytokine production revealed an anti-inflammatory phenotype already at the trauma scene which persisted in the following days, characterized by attenuated TNF-α and IL-6, and increased IL-10 production. Finally, higher concentrations of nDNA and a further decrease of HLA-DRA expression were associated with infections.
Conclusions: Plasma levels of DAMPs are associated with immune suppression, which is apparent within minutes/hours following trauma. Furthermore, aggravated immune suppression during the initial phase following trauma is associated with increased susceptibility towards infections.
Keywords: DAMPs; Immune suppression; Infection; Injury; Trauma.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Comment in
-
Understanding why clinicians should care about danger-associated molecular patterns.Intensive Care Med. 2016 Apr;42(4):611-614. doi: 10.1007/s00134-015-4198-y. Epub 2016 Feb 24. Intensive Care Med. 2016. PMID: 26912314 No abstract available.
-
The role of danger-associated molecular patterns (DAMPs) in trauma and infections.J Thorac Dis. 2016 Jul;8(7):1406-9. doi: 10.21037/jtd.2016.05.22. J Thorac Dis. 2016. PMID: 27500853 Free PMC article. No abstract available.
References
-
- Peden M, McGee K, Sharma G (2002) The injury chart book: a graphical overview of the global burden of injuries. World Health Organization, Geneva
-
- Adminaite D, Allsop R, Jost G (2015) Ranking EU progress on road safety: 9th road safety performance index report. European Transport Safety Council, Brussels
-
- Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, Lopez MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG, Inflammation, Host Response to Injury Large-Scale Collaborative Research Program A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

