The p21-activated kinase, PAK2, is important in the activation of numerous pancreatic acinar cell signaling cascades and in the onset of early pancreatitis events
- PMID: 26912410
- PMCID: PMC4846574
- DOI: 10.1016/j.bbadis.2016.02.008
The p21-activated kinase, PAK2, is important in the activation of numerous pancreatic acinar cell signaling cascades and in the onset of early pancreatitis events
Abstract
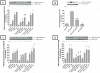
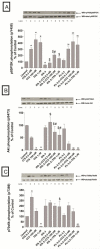
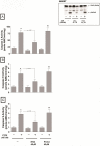
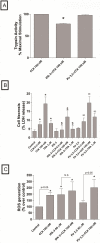
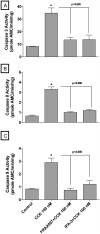
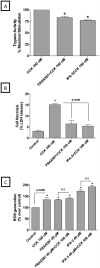
In a recent study we explored Group-1-p21-activated kinases (GP.1-PAKs) in rat pancreatic acini. Only PAK2 was present; it was activated by gastrointestinal-hormones/neurotransmitters and growth factors in a PKC-, Src- and small-GTPase-mediated manner. PAK2 was required for enzyme-secretion and ERK/1-2-activation. In the present study we examined PAK2's role in CCK and TPA-activation of important distal signaling cascades mediating their physiological/pathophysiological effects and analyzed its role in pathophysiological processes important in early pancreatitis. In rat pancreatic acini, PAK2-inhibition by the specific, GP.1.PAK-inhibitor, IPA-3-suppressed cholecystokinin (CCK)/TPA-stimulated activation of focal-adhesion kinases and mitogen-activated protein-kinases. PAK2-inhibition reversed the dual stimulatory/inhibitory effect of CCK/TPA on the PI3K/Akt/GSK-3β pathway. However, its inhibition did not affect PKC activation. PAK2-inhibition protected acini from CCK-induced ROS-generation; caspase/trypsin-activation, important in early pancreatitis; as well as from cell-necrosis. Furthermore, PAK2-inhibition reduced proteolytic-activation of PAK-2p34, which is involved in programmed-cell-death. To ensure that the study did not only rely in the specificity of IPA-3 as a PAK inhibitor, we used two other approaches for PAK inhibition, FRAX597 a ATP-competitive-GP.1-PAKs-inhibitor and infection with a PAK2-dominant negative(DN)-Advirus. Those two approaches confirmed the results obtained with IPA-3. This study demonstrates that PAK2 is important in mediating CCK's effect on the activation of signaling-pathways known to mediate its physiological/pathophysiological responses including several cellular processes linked to the onset of pancreatitis. Our results suggest that PAK2 could be a new, important therapeutic target to consider for the treatment of diseases involving deregulation of pancreatic acinar cells.
Keywords: CCK; Cell death; FRAX597; IPA-3; PAK2; PAK2-DN-advirus; Pancreatic acini; Pancreatitis; Signaling.
Published by Elsevier B.V.
Figures

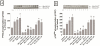
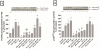


References
-
- Bokoch GM. Biology of the p21-activated kinases. Annu. Rev Biochem. 2003;72:743–781. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

