Effect of Sirolimus on Disease Progression in Patients with Autosomal Dominant Polycystic Kidney Disease and CKD Stages 3b-4
- PMID: 26912555
- PMCID: PMC4858487
- DOI: 10.2215/CJN.09900915
Effect of Sirolimus on Disease Progression in Patients with Autosomal Dominant Polycystic Kidney Disease and CKD Stages 3b-4
Abstract
Background and objectives: The effect of mammalian target of rapamycin (mTOR) inhibitors has never been tested in patients with autosomal dominant polycystic kidney disease (ADPKD) and severe renal insufficiency.
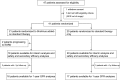
Design, setting, participants, & measurements: In this academic, prospective, randomized, open label, blinded end point, parallel group trial (ClinicalTrials.gov no. NCT01223755), 41 adults with ADPKD, CKD stage 3b or 4, and proteinuria ≤0.5 g/24 h were randomized between September of 2010 and March of 2012 to sirolimus (3 mg/d; serum target levels of 5-10 ng/ml) added on to conventional therapy (n=21) or conventional treatment alone (n=20). Primary outcome was GFR (iohexol plasma clearance) change at 1 and 3 years versus baseline.
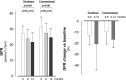
Results: At the 1-year preplanned interim analysis, GFR fell from 26.7±5.8 to 21.3±6.3 ml/min per 1.73 m(2) (P<0.001) and from 29.6±5.6 to 24.9±6.2 ml/min per 1.73 m(2) (P<0.001) in the sirolimus and conventional treatment groups, respectively. Albuminuria (73.8±81.8 versus 154.9±152.9 μg/min; P=0.02) and proteinuria (0.3±0.2 versus 06±0.4 g/24 h; P<0.01) increased with sirolimus. Seven patients on sirolimus versus one control had de novo proteinuria (P=0.04), ten versus three patients doubled proteinuria (P=0.02), 18 versus 11 patients had peripheral edema (P=0.04), and 14 versus six patients had upper respiratory tract infections (P=0.03). Three patients on sirolimus had angioedema, 14 patients had aphthous stomatitis, and seven patients had acne (P<0.01 for both versus controls). Two patients progressed to ESRD, and two patients withdrew because of worsening of proteinuria. These events were not observed in controls. Thus, the independent data and safety monitoring board recommend early trial termination for safety reasons. At 1 year, total kidney volume (assessed by contrast-enhanced computed tomography imaging) increased by 9.0% from 2857.7±1447.3 to 3094.6±1519.5 ml on sirolimus and 4.3% from 3123.4±1695.3 to 3222.6±1651.4 ml on conventional therapy (P=0.12). On follow-up, 37% and 7% of serum sirolimus levels fell below or exceeded the therapeutic range, respectively.
Conclusions: Finding that sirolimus was unsafe and ineffective in patients with ADPKD and renal insufficiency suggests that mTOR inhibitor therapy may be contraindicated in this context.
Keywords: Adult; Humans; Polycystic Kidney, Autosomal Dominant; Prospective Studies; Renal Insufficiency; adverse effects; kidney failure, chronic; proteinuria; randomized controlled trials; sirolimus.
Copyright © 2016 by the American Society of Nephrology.
Figures


References
-
- Gabow PA: Autosomal dominant polycystic kidney disease. N Engl J Med 329: 332–342, 1993 - PubMed
-
- Yamaguchi T, Nagao S, Wallace DP, Belibi FA, Cowley BD, Pelling JC, Grantham JJ: Cyclic AMP activates B-Raf and ERK in cyst epithelial cells from autosomal-dominant polycystic kidneys. Kidney Int 63: 1983–1994, 2003 - PubMed
-
- Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG: PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109: 157–168, 2002 - PubMed
-
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T: The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103: 5466–5471, 2006 - PMC - PubMed
-
- Fingar DC, Blenis J: Target of rapamycin (TOR): An integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151–3171, 2004 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

