Large-scale imaging of cortical dynamics during sensory perception and behavior
- PMID: 26912600
- PMCID: PMC4922607
- DOI: 10.1152/jn.01056.2015
Large-scale imaging of cortical dynamics during sensory perception and behavior
Abstract
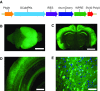
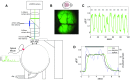
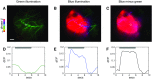
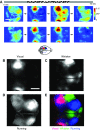
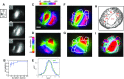
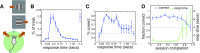
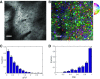
Sensory-driven behaviors engage a cascade of cortical regions to process sensory input and generate motor output. To investigate the temporal dynamics of neural activity at this global scale, we have improved and integrated tools to perform functional imaging across large areas of cortex using a transgenic mouse expressing the genetically encoded calcium sensor GCaMP6s, together with a head-fixed visual discrimination behavior. This technique allows imaging of activity across the dorsal surface of cortex, with spatial resolution adequate to detect differential activity in local regions at least as small as 100 μm. Imaging during an orientation discrimination task reveals a progression of activity in different cortical regions associated with different phases of the task. After cortex-wide patterns of activity are determined, we demonstrate the ability to select a region that displayed conspicuous responses for two-photon microscopy and find that activity in populations of individual neurons in that region correlates with locomotion in trained mice. We expect that this paradigm will be a useful probe of information flow and network processing in brain-wide circuits involved in many sensory and cognitive processes.
Keywords: functional imaging; orientation selectivity; visual cortex.
Copyright © 2016 the American Physiological Society.
Figures


References
-
- Akemann W, Mutoh H, Perron A, Park YK, Iwamoto Y, Knopfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophysiol 108: 2323–2337, 2012. - PubMed
-
- Benson DL, Isackson PJ, Gall CM, Jones EG. Contrasting patterns in the localization of glutamic acid decarboxylase and Ca2+/calmodulin protein kinase gene expression in the rat central nervous system. Neuroscience 46: 825–849, 1992. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

