Identification of the Isoform-specific Interactions between the Tail and the Head of Class V Myosin
- PMID: 26912658
- PMCID: PMC4825024
- DOI: 10.1074/jbc.M115.693762
Identification of the Isoform-specific Interactions between the Tail and the Head of Class V Myosin
Abstract
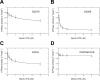
Vertebrates have three isoforms of class V myosin (Myo5), Myo5a, Myo5b, and Myo5c, which are involved in transport of multiple cargoes. It is well established that the motor functions of Myo5a and Myo5b are regulated by a tail inhibition mechanism. Here we found that the motor function of Myo5c was also inhibited by its globular tail domain (GTD), and this inhibition was abolished by high Ca(2+), indicating that the tail inhibition mechanism is conserved in vertebrate Myo5. Interestingly, we found that Myo5a-GTD and Myo5c-GTD were not interchangeable in terms of inhibition of motor function, indicating isoform-specific interactions between the GTD and the head of Myo5. To identify the isoform-specific interactions, we produced a number of Myo5 chimeras by swapping the corresponding regions of Myo5a and Myo5c. We found that Myo5a-GTD, with its H11-H12 loop being substituted with that of Myo5c, was able to inhibit the ATPase activity of Myo5c and that Myo5a-GTD was able to inhibit the ATPase activity of Myo5c-S1 and Myo5c-HMM only when their IQ1 motif was substituted with that of Myo5a. Those results indicate that the H11-H12 loop in the GTD and the IQ1 motif in the head dictate the isoform-specific interactions between the GTD and head of Myo5. Because the IQ1 motif is wrapped by calmodulin, whose conformation is influenced by the sequence of the IQ1 motif, we proposed that the calmodulin bound to the IQ1 motif interacts with the H11-H12 loop of the GTD in the inhibited state of Myo5.
Keywords: ATPase; allosteric regulation; intracellular trafficking; molecular motor; myosin; unconventional myosin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






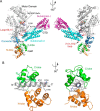
Similar articles
-
Regulation of class V myosin.Cell Mol Life Sci. 2018 Jan;75(2):261-273. doi: 10.1007/s00018-017-2599-5. Epub 2017 Jul 20. Cell Mol Life Sci. 2018. PMID: 28730277 Free PMC article. Review.
-
The Globular Tail Domain of Myosin-5a Functions as a Dimer in Regulating the Motor Activity.J Biol Chem. 2016 Jun 24;291(26):13571-9. doi: 10.1074/jbc.M116.724328. Epub 2016 Apr 28. J Biol Chem. 2016. PMID: 27129208 Free PMC article.
-
Structural insights into the globular tails of the human type v myosins Myo5a, Myo5b, And Myo5c.PLoS One. 2013 Dec 10;8(12):e82065. doi: 10.1371/journal.pone.0082065. eCollection 2013. PLoS One. 2013. PMID: 24339992 Free PMC article.
-
Human myosin-Vc is a novel class V myosin expressed in epithelial cells.J Cell Sci. 2002 Mar 1;115(Pt 5):991-1004. doi: 10.1242/jcs.115.5.991. J Cell Sci. 2002. PMID: 11870218
-
C-terminal isoforms of the myosin heavy chain and smooth muscle function.Comp Biochem Physiol B Biochem Mol Biol. 1997 May;117(1):3-11. doi: 10.1016/s0305-0491(96)00308-2. Comp Biochem Physiol B Biochem Mol Biol. 1997. PMID: 9180009 Review.
Cited by
-
Comparative genomics provides new insights into the remarkable adaptations of the African wild dog (Lycaon pictus).Sci Rep. 2019 Jun 6;9(1):8329. doi: 10.1038/s41598-019-44772-5. Sci Rep. 2019. PMID: 31171819 Free PMC article.
-
Regulation of class V myosin.Cell Mol Life Sci. 2018 Jan;75(2):261-273. doi: 10.1007/s00018-017-2599-5. Epub 2017 Jul 20. Cell Mol Life Sci. 2018. PMID: 28730277 Free PMC article. Review.
-
Autoinhibition and activation mechanisms revealed by the triangular-shaped structure of myosin Va.Sci Adv. 2022 Dec 9;8(49):eadd4187. doi: 10.1126/sciadv.add4187. Epub 2022 Dec 9. Sci Adv. 2022. PMID: 36490350 Free PMC article.
-
Regulation of Myosin-5b by Rab11a and the Rab11 family interacting protein 2.Biosci Rep. 2019 Jan 8;39(1):BSR20181252. doi: 10.1042/BSR20181252. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30545898 Free PMC article.
-
Motor properties of Myosin 5c are modulated by tropomyosin isoforms and inhibited by pentabromopseudilin.Front Physiol. 2024 Mar 28;15:1394040. doi: 10.3389/fphys.2024.1394040. eCollection 2024. Front Physiol. 2024. PMID: 38606007 Free PMC article.
References
-
- Cheney R. E., O'Shea M. K., Heuser J. E., Coelho M. V., Wolenski J. S., Espreafico E. M., Forscher P., Larson R. E., and Mooseker M. S. (1993) Brain myosin-V is a two-headed unconventional myosin with motor activity. Cell 75, 13–23 - PubMed
-
- Rodriguez O. C., and Cheney R. E. (2002) Human myosin-Vc is a novel class V myosin expressed in epithelial cells. J. Cell Sci. 115, 991–1004 - PubMed
-
- Ikebe M. (2008) Regulation of the function of mammalian myosin and its conformational change. Biochem. Biophys. Res. Commun. 369, 157–164 - PubMed
-
- Sellers J. R., Thirumurugan K., Sakamoto T., Hammer J. A. 3rd, and Knight P. J. (2008) Calcium and cargoes as regulators of myosin 5a activity. Biochem. Biophys. Res. Commun. 369, 176–181 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

