Mechanical confinement triggers glioma linear migration dependent on formin FHOD3
- PMID: 26912794
- PMCID: PMC4831879
- DOI: 10.1091/mbc.E15-08-0565
Mechanical confinement triggers glioma linear migration dependent on formin FHOD3
Abstract
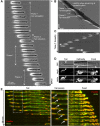
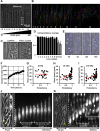
Glioblastomas are extremely aggressive brain tumors with highly invasive properties. Brain linear tracks such as blood vessel walls constitute their main invasive routes. Here we analyze rat C6 and patient-derived glioma cell motility in vitro using micropatterned linear tracks to mimic blood vessels. On laminin-coated tracks (3-10 μm), these cells used an efficient saltatory mode of migration similar to their in vivo migration. This saltatory migration was also observed on larger tracks (50-400 μm in width) at high cell densities. In these cases, the mechanical constraints imposed by neighboring cells triggered this efficient mode of migration, resulting in the formation of remarkable antiparallel streams of cells along the tracks. This motility involved microtubule-dependent polarization, contractile actin bundles and dynamic paxillin-containing adhesions in the leading process and in the tail. Glioma linear migration was dramatically reduced by inhibiting formins but, surprisingly, accelerated by inhibiting Arp2/3. Protein expression and phenotypic analysis indicated that the formin FHOD3 played a role in this motility but not mDia1 or mDia2. We propose that glioma migration under confinement on laminin relies on formins, including FHOD3, but not Arp2/3 and that the low level of adhesion allows rapid antiparallel migration.
© 2016 Monzo et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures







Similar articles
-
Multiple formin proteins participate in glioblastoma migration.BMC Cancer. 2020 Jul 29;20(1):710. doi: 10.1186/s12885-020-07211-7. BMC Cancer. 2020. PMID: 32727404 Free PMC article.
-
Girdin, an actin-binding protein, is critical for migration, adhesion, and invasion of human glioblastoma cells.J Neurochem. 2014 Nov;131(4):457-69. doi: 10.1111/jnc.12831. Epub 2014 Aug 12. J Neurochem. 2014. PMID: 25060559
-
The microtubule inhibiting agent epothilone B antagonizes glioma cell motility associated with reorganization of the actin-binding protein α-actinin 4.Oncol Rep. 2011 Mar;25(3):887-93. doi: 10.3892/or.2011.1145. Epub 2011 Jan 13. Oncol Rep. 2011. PMID: 21234524
-
Formin-binding proteins: modulators of formin-dependent actin polymerization.Biochim Biophys Acta. 2010 Feb;1803(2):174-82. doi: 10.1016/j.bbamcr.2009.06.002. Epub 2009 Jul 7. Biochim Biophys Acta. 2010. PMID: 19589360 Review.
-
Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment.J Natl Cancer Inst. 2007 Nov 7;99(21):1583-93. doi: 10.1093/jnci/djm187. Epub 2007 Oct 30. J Natl Cancer Inst. 2007. PMID: 17971532 Review.
Cited by
-
New advances in probing cell-extracellular matrix interactions.Integr Biol (Camb). 2017 May 22;9(5):383-405. doi: 10.1039/c6ib00251j. Integr Biol (Camb). 2017. PMID: 28352896 Free PMC article. Review.
-
PRG2 and AQPEP are misexpressed in fetal membranes in placenta previa and percreta†.Biol Reprod. 2021 Jul 2;105(1):244-257. doi: 10.1093/biolre/ioab068. Biol Reprod. 2021. PMID: 33982062 Free PMC article.
-
Aligned Collagen Fibers Drive Distinct Traction Force Signatures to Regulate Contact Guidance.ACS Nano. 2025 Aug 26;19(33):30165-30185. doi: 10.1021/acsnano.5c06736. Epub 2025 Aug 14. ACS Nano. 2025. PMID: 40811686 Free PMC article.
-
Targeting the mDia Formin-Assembled Cytoskeleton Is an Effective Anti-Invasion Strategy in Adult High-Grade Glioma Patient-Derived Neurospheres.Cancers (Basel). 2019 Mar 20;11(3):392. doi: 10.3390/cancers11030392. Cancers (Basel). 2019. PMID: 30897774 Free PMC article.
-
Type III Collagen Promotes Pseudopodium-Driven Cell Migration.Chem Bio Eng. 2024 Oct 7;2(2):97-109. doi: 10.1021/cbe.4c00133. eCollection 2025 Feb 27. Chem Bio Eng. 2024. PMID: 40041002 Free PMC article.
References
-
- Agudelo-Garcia PA, De Jesus JK, Williams SP, Nowicki MO, Chiocca EA, Liyanarachchi S, Li PK, Lannutti JJ, Johnson JK, Lawler SE, et al. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia. 2011;13:831–840. - PMC - PubMed
-
- Ayala R, Shu T, Tsai LH. Trekking across the brain: the journey of neuronal migration. Cell. 2007;128:29–43. - PubMed
-
- Azioune A, Carpi N, Tseng Q, Thery M, Piel M. Protein micropatterns: a direct printing protocol using deep UVs. Methods Cell Biol. 2010;97:133–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

