mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle
- PMID: 26912861
- PMCID: PMC4786372
- DOI: 10.1126/science.aad0489
mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle
Abstract
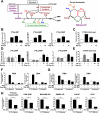
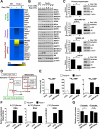
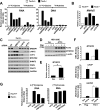
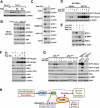
In response to growth signals, mechanistic target of rapamycin complex 1 (mTORC1) stimulates anabolic processes underlying cell growth. We found that mTORC1 increases metabolic flux through the de novo purine synthesis pathway in various mouse and human cells, thereby influencing the nucleotide pool available for nucleic acid synthesis. mTORC1 had transcriptional effects on multiple enzymes contributing to purine synthesis, with expression of the mitochondrial tetrahydrofolate (mTHF) cycle enzyme methylenetetrahydrofolate dehydrogenase 2 (MTHFD2) being closely associated with mTORC1 signaling in both normal and cancer cells. MTHFD2 expression and purine synthesis were stimulated by activating transcription factor 4 (ATF4), which was activated by mTORC1 independent of its canonical induction downstream of eukaryotic initiation factor 2α eIF2α phosphorylation. Thus, mTORC1 stimulates the mTHF cycle, which contributes one-carbon units to enhance production of purine nucleotides in response to growth signals.
Copyright © 2016, American Association for the Advancement of Science.
Figures
Comment in
-
CELL GROWTH. (TORC)ing up purine biosynthesis.Science. 2016 Feb 12;351(6274):670-1. doi: 10.1126/science.aaf1929. Science. 2016. PMID: 26912848 No abstract available.
References
-
- Robitaille AM, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

