Interpreting Clinical Trial Outcomes for Optimal Patient Care: A Survey of Clinicians and Trainees
- PMID: 26913104
- PMCID: PMC4763393
- DOI: 10.4300/JGME-D-15-00137.1
Interpreting Clinical Trial Outcomes for Optimal Patient Care: A Survey of Clinicians and Trainees
Abstract
Background: Evaluation of the clinical importance of outcomes in research studies is an essential element of clinical decision making.
Objective: To understand how clinicians and trainees weigh the importance of different types of clinical outcomes in drug trials.
Methods: A self-administered paper survey contained 4 scenarios asking participants to rate (1, "no proof" to 10, "good proof") the extent to which 4 study outcomes provided "proof that the new drug might help people." Outcomes included (1) a surrogate outcome; (2) a surrogate-enriched composite outcome; (3) stroke mortality; and (4) all-cause mortality. The primary study metrics were mean ratings for each of the 4 outcome types, and the proportion ranking outcome importance of all-cause mortality > stroke mortality > surrogate-enriched composite or surrogate alone.
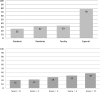
Results: A convenience sample of 549 clinicians and trainees at 2 medical centers completed the survey (response rate: 87% medical students, 80% internal medicine residents, 69% general medicine faculty, and 41% physician experts). The surrogate-enriched composite outcome and stroke mortality were rated the most important evidence for benefit (6.6 and 6.4, respectively), with all-cause mortality and a surrogate outcome being rated significantly lower (5.2 and 4.6, respectively). In addition, 48% of clinicians rated improvement in all-cause mortality as more valuable than an improvement in a surrogate marker. Only 21% rated all-cause mortality as more valuable than a surrogate-enriched composite outcome.
Conclusions: These findings raise concerns that clinicians and trainees may not interpret trial evidence in a way that promotes the best care for patients.
Conflict of interest statement
Conflict of interest: The authors declare they have no competing interests.
Figures
Comment in
-
Blinding Them With Science? Evidence-Based Medicine as a Barrier to Health Care Value.J Grad Med Educ. 2016 Feb;8(1):106-8. doi: 10.4300/JGME-D-15-00570.1. J Grad Med Educ. 2016. PMID: 26913115 Free PMC article. No abstract available.
References
-
- Woloshin W, Schwartz LM, Welch HG. Know Your Chances: Understanding Health Statistics. 1st ed. Berkeley: University of California Press;; 2008. - PubMed
-
- Guyatt G. JAMA's Users' Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. New York, NY: McGraw-Hill Medical;; 2008.
-
- Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94(3):167–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

