Revealing T-Tubules in Striated Muscle with New Optical Super-Resolution Microscopy Techniquess
- PMID: 26913143
- PMCID: PMC4748971
- DOI: 10.4081/ejtm.2015.4747
Revealing T-Tubules in Striated Muscle with New Optical Super-Resolution Microscopy Techniquess
Abstract
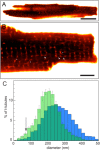
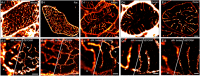
The t-tubular system plays a central role in the synchronisation of calcium signalling and excitation-contraction coupling in most striated muscle cells. Light microscopy has been used for imaging t-tubules for well over 100 years and together with electron microscopy (EM), has revealed the three-dimensional complexities of the t-system topology within cardiomyocytes and skeletal muscle fibres from a range of species. The emerging super-resolution single molecule localisation microscopy (SMLM) techniques are offering a near 10-fold improvement over the resolution of conventional fluorescence light microscopy methods, with the ability to spectrally resolve nanometre scale distributions of multiple molecular targets. In conjunction with the next generation of electron microscopy, SMLM has allowed the visualisation and quantification of intricate t-tubule morphologies within large areas of muscle cells at an unprecedented level of detail. In this paper, we review recent advancements in the t-tubule structural biology with the utility of various microscopy techniques. We outline the technical considerations in adapting SMLM to study t-tubules and its potential to further our understanding of the molecular processes that underlie the sub-micron scale structural alterations observed in a range of muscle pathologies.
Keywords: cardiac muscle; excitation-contraction coupling; horse; human; mouse; rabbit; rat; skeletal muscle; super-resolution microscopy; t-tubules.
Figures


References
-
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 1997;77:699-729. Epub 1997/07/01. - PubMed
-
- Asghari P, Scriven DR, Sanatani S, et al. Nonuniform and variable arrangements of ryanodine receptors within mammalian ventricular couplons. Circ Res 2014;115:252-62. Epub 2014/05/03. - PubMed
-
- Huxley AF. Local activation of striated muscle from the frog and the crab. J Physiol 1957;135:17-8P. Epub 1957/01/23. - PubMed
-
- Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol 2011;50:451-9. Epub 2010/12/15. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

