Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products
- PMID: 26913160
- PMCID: PMC4748978
- DOI: 10.4081/ejtm.2015.5272
Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products
Abstract
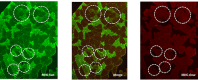
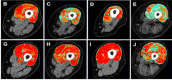
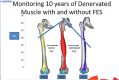
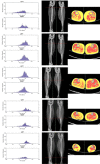
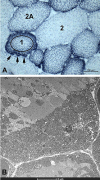
There is something in our genome that dictates life expectancy and there is nothing that can be done to avoid this; indeed, there is not yet any record of a person who has cheated death. Our physical prowess can vacillate substantially in our lifetime according to our activity levels and nutritional status and we may fight aging, but we will inevitably lose. We have presented strong evidence that the atrophy which accompanies aging is to some extent caused by loss of innervation. We compared muscle biopsies of sedentary seniors to those of life long active seniors, and show that these groups indeed have a different distribution of muscle fiber diameter and fiber type. The senior sportsmen have many more slow fiber-type groupings than the sedentary people which provides strong evidence of denervation-reinnervation events in muscle fibers. It appears that activity maintains the motoneurons and the muscle fibers. Premature or accelerated aging of muscle may occur as the result of many chronic diseases. One extreme case is provided by irreversible damage of the Conus and Cauda Equina, a spinal cord injury (SCI) sequela in which the human leg muscles may be completely and permanently disconnected from the nervous system with the almost complete disappearance of muscle fibers within 3-5 years from SCI. In cases of this extreme example of muscle degeneration, we have used 2D Muscle Color CT to gather data supporting the idea that electrical stimulation of denervated muscles can retain and even regain muscle. We show here that, if people are compliant, atrophy can be reversed. A further example of activity-related muscle adaptation is provided by the fact that mitochondrial distribution and density are significantly changed by functional electrical stimulation in horse muscle biopsies relative to those not receiving treatment. All together, the data indicate that FES is a good way to modify behaviors of muscle fibers by increasing the contraction load per day. Indeed, it should be possible to defer the muscle decline that occurs in aging people and in those who have become unable to participate in physical activities. Thus, FES should be considered for use in rehabilitation centers, nursing facilities and in critical care units when patients are completely inactive even for short periods of time.
Keywords: Muscle power; aging decay; equine muscle spasm; h-b FES-induced muscle recovery; long-term denervated muscles; master athletes; muscle denervation/reinnervation; subsarcolemmal mitochondria; type groupings.
Figures

References
-
- Hill AV. The physiological basis of athletic records. The Scientific Monthly 1925;2:409–428.
-
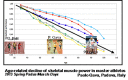
- Gava P, Kern H, Carraro U. Age-associated power decline from running, jumping, and throwing male masters world records. Exp Aging Res. 2015;41(2):115–35. doi: 10.1080/0361073X.2015.1001648. - PubMed
-
- Mosole S, Carraro U, Kern H, et al. Long-term high-level exercise promotes muscle reinnervation with age. J Neuropathol Exp Neurol 2014;73:284–94. doi: 10.1097/NEN.0000000000 000032. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

