Enhanced biocompatibility of CD47-functionalized vascular stents
- PMID: 26914699
- PMCID: PMC5304342
- DOI: 10.1016/j.biomaterials.2016.02.008
Enhanced biocompatibility of CD47-functionalized vascular stents
Abstract
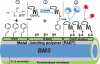
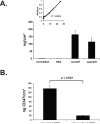
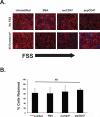
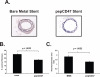
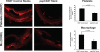
The effectiveness of endovascular stents is hindered by in-stent restenosis (ISR), a secondary re-obstruction of treated arteries due to unresolved inflammation and activation of smooth muscle cells in the arterial wall. We previously demonstrated that immobilized CD47, a ubiquitously expressed transmembrane protein with an established role in immune evasion, can confer biocompatibility when appended to polymeric surfaces. In present studies, we test the hypothesis that CD47 immobilized onto metallic surfaces of stents can effectively inhibit the inflammatory response thus mitigating ISR. Recombinant CD47 (recCD47) or a peptide sequence corresponding to the Ig domain of CD47 (pepCD47), were attached to the surfaces of both 316L-grade stainless steel foils and stents using bisphosphonate coordination chemistry and thiol-based conjugation reactions to assess the anti-inflammatory properties of CD47-functionalized surfaces. Initial in vitro and ex vivo analysis demonstrated that both recCD47 and pepCD47 significantly reduced inflammatory cell attachment to steel surfaces without impeding on endothelial cell retention and expansion. Using a rat carotid stent model, we showed that pepCD47-functionalized stents prevented fibrin and platelet thrombus deposition, inhibited inflammatory cell attachment, and reduced restenosis by 30%. It is concluded that CD47-modified stent surfaces mitigate platelet and inflammatory cell attachment, thereby disrupting ISR pathophysiology.
Keywords: Bare metal stents; Bioactive surface coating; Inflammation; Platelets; Restenosis; Signal regulatory protein alpha.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures
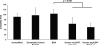
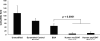
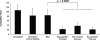

References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. - PubMed
-
- Butt M, Connolly D, Lip GY. Drug-eluting stents: a comprehensive appraisal. Future cardiology. 2009;5:141–57. - PubMed
-
- Simard T, Hibbert B, Ramirez FD, Froeschl M, Chen YX, O'Brien ER. The evolution of coronary stents: a brief review. Can J Cardiol. 2014;30:35–45. - PubMed
-
- Rathore S, Terashima M, Katoh O, Matsuo H, Tanaka N, Kinoshita Y, et al. Predictors of angiographic restenosis after drug eluting stents in the coronary arteries: contemporary practice in real world patients. EuroIntervention. 2009;5:349–54. - PubMed
-
- Jaffe R, Strauss BH. Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

