Cost-Effectiveness of Vaccinating Immunocompetent ≥65 Year Olds with the 13-Valent Pneumococcal Conjugate Vaccine in England
- PMID: 26914907
- PMCID: PMC4767406
- DOI: 10.1371/journal.pone.0149540
Cost-Effectiveness of Vaccinating Immunocompetent ≥65 Year Olds with the 13-Valent Pneumococcal Conjugate Vaccine in England
Abstract
Background: Recently a large clinical trial showed that the use of 13-valent pneumococcal conjugate vaccine (PCV13) among immunocompetent individuals aged 65 years and over was safe and efficacious. The aim of this study was to assess the cost-effectiveness of vaccinating immunocompetent 65 year olds with PCV13 vaccine in England. England is a country with universal childhood pneumococcal conjugate vaccination programme in place (7-valent (PCV7) since 2006 and PCV13 since 2010), as well as a 23-valent pneumococcal polysaccharide (PPV23) vaccination programme targeting clinical risk-groups and those ≥65 years.
Method: A static cohort cost-effectiveness model was developed to follow a cohort of 65 year olds until death, which will be vaccinated in the autumn of 2016 with PCV13. Sensitivity analysis was performed to test the robustness of the results.
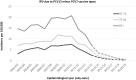
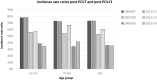
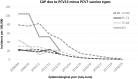
Results: The childhood vaccination programme with PCV7 has induced herd protection among older unvaccinated age groups, with a resultant low residual disease burden caused by PCV7 vaccine types. We show similar herd protection effects for the 6 additional serotypes included in PCV13, and project a new low post-introduction equilibrium of vaccine-type disease in 2018/19. Applying these incidence projections for both invasive disease and community-acquired pneumonia (CAP), and using recent measures of vaccine efficacy against these endpoints for ≥65 year olds, we estimate that vaccination of a cohort of immunocompetent 65 year olds with PCV13 would directly prevent 26 cases of IPD, 69 cases of CAP and 15 deaths. The associated cost-effectiveness ratio is £257,771 per QALY gained (using list price of £49.10 per dose and £7.51 administration costs) and is therefore considered not cost-effective. To obtain a cost-effective programme the price per dose would need to be negative. The results were sensitive to disease incidence, waning vaccine protection and case fatality rate; despite this, the overall conclusion was robust.
Conclusions: Vaccinating immunocompetent individuals aged ≥65 years with PCV13 is efficacious. However the absolute incidence of vaccine-type disease will likely become very low due to wider benefits of the childhood PCV13 vaccination programme, such that a specific PCV13 vaccination programme targeting the immunocompetent elderly would not be cost-effective.
Conflict of interest statement
Figures


References
-
- Andrews N, Waight PA, George RC, Slack MPE, Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. Elsevier; 2012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

