Large animal models of cardiovascular disease
- PMID: 26914991
- PMCID: PMC4834612
- DOI: 10.1002/cbf.3173
Large animal models of cardiovascular disease
Abstract
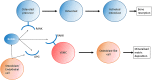
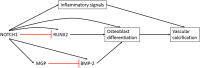
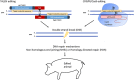
The human cardiovascular system is a complex arrangement of specialized structures with distinct functions. The molecular landscape, including the genome, transcriptome and proteome, is pivotal to the biological complexity of both normal and abnormal mammalian processes. Despite our advancing knowledge and understanding of cardiovascular disease (CVD) through the principal use of rodent models, this continues to be an increasing issue in today's world. For instance, as the ageing population increases, so does the incidence of heart valve dysfunction. This may be because of changes in molecular composition and structure of the extracellular matrix, or from the pathological process of vascular calcification in which bone-formation related factors cause ectopic mineralization. However, significant differences between mice and men exist in terms of cardiovascular anatomy, physiology and pathology. In contrast, large animal models can show considerably greater similarity to humans. Furthermore, precise and efficient genome editing techniques enable the generation of tailored models for translational research. These novel systems provide a huge potential for large animal models to investigate the regulatory factors and molecular pathways that contribute to CVD in vivo. In turn, this will help bridge the gap between basic science and clinical applications by facilitating the refinement of therapies for cardiovascular disease.
Keywords: Marfan syndrome; aortic stenosis; calcific aortic valve disease; cardiovascular disease; genetic engineering; large animal models; vascular calcification.
Copyright © 2016 John Wiley & Sons, Ltd.
Figures

References
-
- Solomon EP, Berg LR, Martin DW. Biology. Thomson‐Brooks/Cole, London: Belmont, Calif., 2008.
-
- Ross MH, Pawlina W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology. Lippincott Williams & Wilkins: Philadelphia, Pa.; London, 2011.
-
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095–2128. doi:10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics—2014 update a report from the American Heart Association. Circulation 2014; 129: 399–410. doi:10.1161/01.cir.0000442015.53336.12. - DOI - PubMed
-
- WHO . World Health Statistics 2014. World Health Organization: Geneva, 2014.
Publication types
MeSH terms
Grants and funding
- BBS/E/D/20211553/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/R/00001608/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F023928/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004316/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J01446X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

