Cell Assembly Dynamics of Sparsely-Connected Inhibitory Networks: A Simple Model for the Collective Activity of Striatal Projection Neurons
- PMID: 26915024
- PMCID: PMC4767417
- DOI: 10.1371/journal.pcbi.1004778
Cell Assembly Dynamics of Sparsely-Connected Inhibitory Networks: A Simple Model for the Collective Activity of Striatal Projection Neurons
Abstract
Striatal projection neurons form a sparsely-connected inhibitory network, and this arrangement may be essential for the appropriate temporal organization of behavior. Here we show that a simplified, sparse inhibitory network of Leaky-Integrate-and-Fire neurons can reproduce some key features of striatal population activity, as observed in brain slices. In particular we develop a new metric to determine the conditions under which sparse inhibitory networks form anti-correlated cell assemblies with time-varying activity of individual cells. We find that under these conditions the network displays an input-specific sequence of cell assembly switching, that effectively discriminates similar inputs. Our results support the proposal that GABAergic connections between striatal projection neurons allow stimulus-selective, temporally-extended sequential activation of cell assemblies. Furthermore, we help to show how altered intrastriatal GABAergic signaling may produce aberrant network-level information processing in disorders such as Parkinson's and Huntington's diseases.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

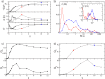
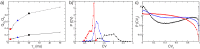
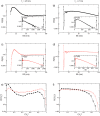
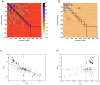

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

