Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier
- PMID: 26915097
- PMCID: PMC4767601
- DOI: 10.1371/journal.ppat.1005442
Nitric Oxide Protects against Infection-Induced Neuroinflammation by Preserving the Stability of the Blood-Brain Barrier
Abstract
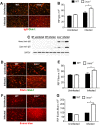
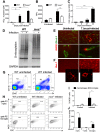
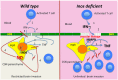
Nitric oxide (NO) generated by inducible NO synthase (iNOS) is critical for defense against intracellular pathogens but may mediate inflammatory tissue damage. To elucidate the role of iNOS in neuroinflammation, infections with encephalitogenic Trypanosoma brucei parasites were compared in inos(-/-) and wild-type mice. Inos(-/-) mice showed enhanced brain invasion by parasites and T cells, and elevated protein permeability of cerebral vessels, but similar parasitemia levels. Trypanosome infection stimulated T cell- and TNF-mediated iNOS expression in perivascular macrophages. NO nitrosylated and inactivated pro-inflammatory molecules such as NF-κΒp65, and reduced TNF expression and signalling. iNOS-derived NO hampered both TNF- and T cell-mediated parasite brain invasion. In inos(-/-) mice, TNF stimulated MMP, including MMP9 activity that increased cerebral vessel permeability. Thus, iNOS-generated NO by perivascular macrophages, strategically located at sites of leukocyte brain penetration, can serve as a negative feed-back regulator that prevents unlimited influx of inflammatory cells by restoring the integrity of the blood-brain barrier.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Thiel VE, Audus KL Nitric oxide and blood-brain barrier integrity. Antioxid Redox Signal. 2001; 3: 273–278. - PubMed
-
- Smith KJ, Lassmann H The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002; 1: 232–241. - PubMed
-
- Cauwels A, Van Molle W, Janssen B, Everaerdt B, Huang P, Fiers W, et al. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity. 2000; 13: 223–231. - PubMed
-
- Dumas M, Bisser S (1999) Clinical aspects of human African trypanosomiasis In: Dumas M, Bouteille B, Buguet A, editors. Progress in human African trypanosomiasis, sleeping sickness. Paris: Springer; pp. 215–233.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

