Topical treatments for scalp psoriasis
- PMID: 26915340
- PMCID: PMC8697570
- DOI: 10.1002/14651858.CD009687.pub2
Topical treatments for scalp psoriasis
Abstract
Background: People with chronic plaque psoriasis often have lesions on the scalp. Hair makes the scalp difficult to treat and the adjacent facial skin is particularly sensitive to topical treatments.
Objectives: To assess the efficacy and safety of topical treatments for scalp psoriasis.
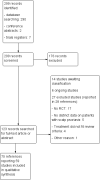
Search methods: We searched the following databases up to August 2015: the Cochrane Skin Group Specialised Register, CENTRAL (2015, Issue 7), MEDLINE (from 1946), EMBASE (from 1974) and LILACS (from 1982). We also searched five trials registers, screened abstracts of six psoriasis-specific conferences and checked the reference lists of included studies for further references to relevant randomised controlled trials.
Selection criteria: Randomised controlled trials (RCTs) with a parallel-group, cross-over or within-patient design of topical treatments for people of all ages with scalp psoriasis.
Data collection and analysis: Two authors independently carried out study selection, data extraction and 'Risk of bias' assessment. Disagreements were settled by reference to a third author.To assess the quality of evidence, we focused on the following outcomes: 'clearance' or 'response' as assessed by the investigator global assessment (IGA), improvement in quality of life, adverse events requiring withdrawal of treatment and 'response' as assessed by the patient global assessment (PGA).We expressed the results of the single studies as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes, and mean differences (MD) with 95% CI for continuous outcomes. If studies were sufficiently homogeneous, we meta-analysed the data by using the random-effects model. Where it was not possible to calculate a point estimate for a single study, we described the data qualitatively. We also presented the number needed to treat to benefit (NNTB).We categorised topical corticosteroids according to the German classification of corticosteroid potency as mild, moderate, high and very high.
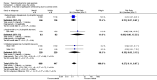
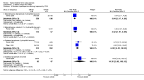
Main results: We included 59 RCTs with a total of 11,561 participants. Thirty studies were either conducted or sponsored by the manufacturer of the study medication. The risk of bias varied considerably among the included studies. For instance, most authors did not state the randomisation method and few addressed allocation concealment. Most findings were limited to short-term treatments, since most studies were conducted for less than six months. Only one trial investigated long-term therapy (12 months). Although we found a wide variety of different interventions, we limited the grading of the quality of evidence to three major comparisons: steroid versus vitamin D, two-compound combination of steroid and vitamin D versus steroid monotherapy and versus vitamin D.In terms of clearance, as assessed by the IGA, steroids were better than vitamin D (RR 1.82; 95% CI 1.52 to 2.18; four studies, 2180 participants, NNTB = 8; 95% CI 7 to 11; moderate quality evidence). Statistically, the two-compound combination was superior to steroid monotherapy, however the additional benefit was small (RR 1.22; 95% CI 1.08 to 1.36; four studies, 2474 participants, NNTB = 17; 95% CI 11 to 41; moderate quality evidence). The two-compound combination was more effective than vitamin D alone (RR 2.28; 95% CI 1.87 to 2.78; four studies, 2008 participants, NNTB = 6; 95% CI 5 to 7; high quality evidence).In terms of treatment response, as assessed by the IGA, corticosteroids were more effective than vitamin D (RR 2.09; 95% CI 1.80 to 2.41; three studies, 1827 participants; NNTB = 4; 95% CI 4 to 5; high quality evidence). The two-compound combination was better than steroid monotherapy, but the additional benefit was small (RR 1.15; 95% CI 1.06 to 1.25; three studies, 2444 participants, NNTB = 13; 95% CI 9 to 24; moderate quality evidence). It was also more effective than vitamin D alone (RR 2.31; 95% CI 1.75 to 3.04; four studies, 2222 participants, NNTB = 3; 95% CI 3 to 4; moderate quality evidence).Reporting of quality of life data was poor and data were insufficient to be included for meta-analysis.Steroids caused fewer withdrawals due to adverse events than vitamin D (RR 0.22; 95% CI 0.11 to 0.42; four studies, 2291 participants; moderate quality evidence). The two-compound combination and steroid monotherapy did not differ in the number of adverse events leading withdrawal (RR 0.88; 95% CI 0.42 to 1.88; three studies, 2433 participants; moderate quality evidence). The two-compound combination led to fewer withdrawals due to adverse events than vitamin D (RR 0.19; 95% CI 0.11 to 0.36; three studies, 1970 participants; high quality evidence). No study reported the type of adverse event requiring withdrawal.In terms of treatment response, as assessed by the PGA, steroids were more effective than vitamin D (RR 1.48; 95% CI 1.28 to 1.72; three studies, 1827 participants; NNTB = 5; 95% CI 5 to 7; moderate quality evidence). Statistically, the two-compound combination was better than steroid monotherapy, however the benefit was not clinically important (RR 1.13; 95% CI 1.06 to 1.20; two studies, 2226 participants; NNTB = 13; 95% CI 9 to 26; high quality evidence). The two-compound combination was more effective than vitamin D (RR 1.76; 95% CI 1.46 to 2.12; four studies, 2222 participants; NNTB = 4; 95% CI 3 to 6; moderate quality evidence).Common adverse events with these three interventions were local irritation, skin pain and folliculitis. Systemic adverse events were rare and probably not drug-related.In addition to the results of the major three comparisons we found that the two-compound combination, steroids and vitamin D monotherapy were more effective than the vehicle. Steroids of moderate, high and very high potency tended to be similarly effective and well tolerated. There are inherent limitations in this review concerning the evaluation of salicylic acid, tar, dithranol or other topical treatments.
Authors' conclusions: The two-compound combination as well as corticosteroid monotherapy were more effective and safer than vitamin D monotherapy. Given the similar safety profile and only slim benefit of the two-compound combination over the steroid alone, monotherapy with generic topical steroids may be fully acceptable for short-term therapy.Future RCTs should investigate how specific therapies improve the participants' quality of life. Long-term assessments are needed (i.e. 6 to 12 months).
Conflict of interest statement
AN has received honoraria as a speaker in educational activities with direct or indirect sponsoring from Bayer, Pfizer, Novartis, Abbot and Biogen Idec. This has been in the last three years and is limited to the companies with an interest in psoriasis treatment.
The dEBM (JGS, SR, RNW and AJ) has received research grants from Pfizer, Biogen Idec, GSK and Merz.
JS received funding for investigator‐initiated research from Novartis, MSD, Pfizer, Alk and Sanofi.
CS has no conflicts of interest to declare.
A clinical referee, who wishes to remain anonymous: "I received speaker's honoraria or fees as investigator of clinical trials from Leo Pharma and Galderma."
Figures






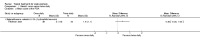
































































Update of
- doi: 10.1002/14651858.CD009687
References
References to studies included in this review
Andres 2006 {published data only}
-
- Andres P, Poncet M, Farzaneh S, Soto P. Short‐term safety assessment of clobetasol propionate 0.05% shampoo: hypothalamic‐pituitary‐adrenal axis suppression, atrophogenicity, and ocular safety in subjects with scalp psoriasis. Journal of Drugs in Dermatology 2006;5(4):328‐32. [MEDLINE: ] - PubMed
Barrett 2005 {published data only (unpublished sought but not used)}
-
- Barrett C, Lowson D, Blades KJ. Limited benefit of combined use of tar‐based shampoo with 50 mug/ml calcipotriol solution in scalp psoriasis. Journal of Dermatological Treatment 2005;16(3):175. [EMBASE: 2005434030] - PubMed
Bergstrom 2003 {published data only}
-
- Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single‐blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis 2003;72(5):407‐11. [MEDLINE: ] - PubMed
Breneman 1992 {published data only}
-
- Breneman DL, Davis MB, Berger V, Chaney R. A double‐blind trial comparing the efficacy and safety of augmented betamethasone dipropionate lotion with fluocinonide solution in the treatment of severe scalp psoriasis. Journal of Dermatological Treatment 1992;3(1):19‐21. [EMBASE: 1992153744]
Buckley 2008 {published data only (unpublished sought but not used)}
-
- Buckley C, Hoffmann V. Calcipotriol plus betamethasone dipropionate gel is effective and safe in the treatment of scalp psoriasis (a phase II study). (Abstract P06.57). Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):175.
-
- Buckley C, Hoffmann V, Shapiro J, Saari S, Cambazard F, Milsgaard M. Calcipotriol plus betamethasone dipropionate scalp formulation is effective and well tolerated in the treatment of scalp psoriasis: a phase II study. Dermatology 2008;217(2):107‐13. [MEDLINE: ] - PubMed
Curley 1990 {published data only}
-
- Curley RK, Vickers CF, Norris T, Glover DR. A comparative study of betamethasone dipropionate with salicylic acid and betamethasone valerate for the treatment of steroid‐responsive dermatoses of the scalp. Journal of Dermatological Treatment 1990;1(4):203‐6. [EMBASE: 1990343409]
De Cuyper 1995 {published data only}
-
- Cuyper C, Greef H, Brassinne M, Delescule J, Derumeuax L, Heenen M, et al. A randomized single‐blind study to compare hydrocortisone 17‐butyrate 0.1% emulsion versus betamethasone 17,21‐dipropionate 0.05% lotion in the treatment of patients suffering from psoriasis of the scalp. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S104‐5.
Duweb 2000 {published data only}
-
- Duweb GA, Abuzariba O, Rahim M, al‐Taweel M, Abdulla SA. Scalp psoriasis: topical calcipotriol 50 micrograms/g/ml solution vs. betamethasone valerate 1% lotion. International Journal of Clinical Pharmacology Research 2000;20(3‐4):65‐8. [MEDLINE: ] - PubMed
Ellis 1988 {published data only}
-
- Ellis CN, Horwitz SN, Menter A. Amcinonide lotion 0.1% in the treatment of patients with psoriasis of the scalp. Current Therapeutic Research ‐ Clinical and Experimental 1988;44(2):315‐24. [EMBASE: 1989002095]
Ellis 1989 {published data only}
-
- Ellis CN, Menter MA. A randomized, blinded comparison of amcinonide lotion and fluocinonide solution in patients with psoriasis of the scalp. Current Therapeutic Research ‐ Clinical and Experimental 1989;46(3):471‐7. [EMBASE: 1989232557]
Feldman 2001 {published data only}
-
- Feldman SR, Ravis SM, Fleischer AB, McMichael A, Jones E, Kaplan R, et al. Betamethasone valerate in foam vehicle is effective with both daily and twice a day dosing: a single‐blind, open‐label study in the treatment of scalp psoriasis. Journal of Cutaneous Medicine & Surgery 2001;5(5):386‐9. [MEDLINE: ] - PubMed
Feldman 2013 {published data only}
-
- Feldman SR, Mills M, Brundage T, Eastman WJ. A multicenter, randomized, double‐blind study of the efficacy and safety of calcipotriene foam, 0.005%, vs vehicle foam in the treatment of plaque‐type psoriasis of the scalp. Journal of Drugs in Dermatology 2013;12(3):300‐6. [MEDLINE: ] - PubMed
Franz 1999 {published data only}
-
- Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. International Journal of Dermatology 1999;38(8):628‐32. [MEDLINE: ] - PubMed
Franz 2000 {published data only}
-
- Franz TJ, Parsell DA, Myers JA, Hannigan JF. Clobetasol propionate foam 0.05%: a novel vehicle with enhanced delivery. International Journal of Dermatology 2000;39(7):535‐8. [MEDLINE: ] - PubMed
Fredriksson 1976 {published data only}
-
- Fredriksson T. A clinical comparison of 3 corticosteroid alcoholic solutions in the treatment of psoriasis of the scalp. Pharmatherapeutica 1976;1(4):252‐6. [EMBASE: 0977155168]
Gip 1981 {published data only}
-
- Gip L. Hydrocortisone 17‐butyrate 0.1% cream and fluocinolone acetonide 0.025% cream: a double‐blind comparison in patients suffering from psoriasis of the scalp. Current Therapeutic Research ‐ Clinical and Experimental 1981;29(1 II):198‐201. [EMBASE: 1981110060]
Green 1994 {published data only}
-
- Green C, Ganpule M, Harris D, Kavanagh G, Kennedy C, Mallett R, et al. Comparative effects of calcipotriol (MC903) solution and placebo (vehicle of MC903) in the treatment of psoriasis of the scalp. British Journal of Dermatology 1994;130(4):483‐7. [MEDLINE: ] - PubMed
Griffiths 2006 {published data only}
-
- Griffiths C, Barker J, Finlay A, Mizzi F, Arsonnaud S. A new formulation of clobetasol proprionate is more effective and safer than 1% tar shampoo in scalp. Annales de Dermatologie et de Vénéréologie 2002;129:1S740.
-
- Griffiths C, Barker J, Finlay A, Mizzi F, Arsonnaud S. A new formulation of clobetasol proprionate is safe and more effective than 1% tar shampoo in scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 2002;16(Suppl 1):277.
-
- Griffiths CE, Finlay AY, Fleming CJ, Barker JN, Mizzi F, Arsonnaud S. A randomized, investigator‐masked clinical evaluation of the efficacy and safety of clobetasol propionate 0.05% shampoo and tar blend 1% shampoo in the treatment of moderate to severe scalp psoriasis. Journal of Dermatological Treatment 2006;17(2):90‐5. [MEDLINE: ] - PubMed
Harris 1972 {published data only}
-
- Harris JJ. A national double‐blind clinical trial of a new corticosteroid lotion: a 12‐investigator cooperative analysis. Current Therapeutic Research, Clinical & Experimental 1972;14(9):638‐46. [MEDLINE: ] - PubMed
He 2008 {published data only}
-
- He YL, Guo ZP. Clinical efficacy of 0.1% tacrolimus ointment on plaque psoriasis of scalp and face. Journal of Clinical Dermatology 2008;37(4):254‐5. [EMBASE: 2008250949]
Hillstrom 1978 {published data only}
-
- Hillstrom L, Pettersson L. Studies with betamethasone dipropionate lotion with salicylic acid (Diprosalic) in psoriasis and seborrhoic eczema of the scalp. Current Therapeutic Research ‐ Clinical and Experimental 1978;24(1):46‐50. [EMBASE: 0979244015]
Hillstrom 1982 {published data only}
-
- Hillstrom L, Pettersson L, Svensson L. Comparison of betamethasone dipropionate lotion with salicylic acid (Diprosalic(TM)) and clobetasol propionate lotion (Dermovate(TM)) in the treatment of psoriasis of the scalp. Journal of International Medical Research 1982;10(6):419‐22. [MEDLINE: ] - PubMed
Hillstrom 1984 {published data only}
-
- Hillstrom L. Comparison of topical treatment with desoxymethasone solution 0.25% with salicylic acid 1% and betamethasone valerate solution 0.1% in patients with psoriasis of the scalp. Journal of International Medical Research 1984;12(3):170‐3. [MEDLINE: ] - PubMed
Housman 2002 {published data only (unpublished sought but not used)}
-
- Housman TS, McMichael AJ, Mellen BG, Derrow AE, McCarty MA, Feldman SR. Use of 0.12% betamethasone valerate foam vs 0.01% fluocinolone acetonide topical oil to treat scalp psoriasis: quantitative assessment of patient preference and treatment efficacy. Cosmetic Dermatology 2002;15(11):27‐30. [EMBASE: 2006007462]
-
- Housman TS, McMichael AJ, Mellen BG, Derrow E, McCarty MA, Feldman SR. Betamethasone valerate foam vs. fluocinolone acetonide topical oil in the treatment of scalp psoriasis: quantitative assessment of patients' preference and efficacy (Abstract 203). Journal of Investigative Dermatology 2002;119(1):241.
Jarratt 1991 {published data only}
-
- Jarratt M, Davis JG, Giltner MP, Jones ML, Peets EA. Comparative studies of augmented betamethasone dipropionate lotion 0.05% and clobetasol propionate solution 0.05%: Correlation of the vasoconstriction assay and clinical activity in scalp psoriasis. Advances in Therapy 1991;8(2):103‐11. [EMBASE: 1991176103]
Jarratt 2004 {published data only}
-
- Claudel J‐P, Fredon L, Maccari F, Savary J. Nantes, March 26, 2009: A new response to psoriasis of the scalp: Clobex®, first corticosteroid shampoo [Nantes, le 26 mars 2009: Une nouvelle réponse au psoriasis du cuir chevelu: Clobex®, premier shampooing corticoïde]. Nouvelles Dermatologiques 2009;28(6 Pt 1):341‐4. [EMBASE: 2009457722]
-
- Jarrat M, Beneman D, Gottlieb A, Poulin Y, Foley V. Efficacy and safety comparison of clobetasol proprionate shampoo 0.05% and vehicle in scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 2002;16(Suppl 1):282‐3.
-
- Jarratt M, Breneman D, Gottlieb AB, Poulin Y, Liu Y, Foley V. Clobetasol propionate shampoo 0.05%: a new option to treat patients with moderate to severe scalp psoriasis. Journal of Drugs in Dermatology 2004;3(4):367‐73. [MEDLINE: ] - PubMed
Jemec 2008 {published data only}
-
- Jemec GB, Ganslandt C, Ortonne JP, Poulin Y, Burden AD, Unamuno P, et al. A new scalp formulation of calcipotriene plus betamethasone compared with its active ingredients and the vehicle in the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. Journal of the American Academy of Dermatology 2008;59(3):455‐63. [MEDLINE: ] - PubMed
-
- Jemec GBE, Burden D, Poulin Y, Ortonne JP. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients and the vehicle (Abstract P2733). Journal of the American Academy of Dermatology 2007;56(2):AB182. - PubMed
Josse 2005 {published data only}
-
- Josse M, Mengeaud V, Khemis A. Efficacy of a new keratolytic shampoo containing glycolic acid and ictyol for the treatment of scalp psoriasis: results of a comparative study (Abstract P1604). Journal of the American Academy of Dermatology 2006;54(3 Suppl):AB128.
-
- Josse M, Mengeaud V, Khemis A, Ortonne JP. Efficacy of a new keratolytic shampoo containing glycolic acid and ictyol for the treatment of scalp psoriasis ‐ results of a comparative study (Abstract P16.34). Journal of the European Academy of Dermatology & Venereology 2005;19(Suppl 2):391.
Katz 1995 {published data only}
-
- Katz HI, Lindholm JS, Weiss JS, Shavin JS, Morman M, Bressinck R, et al. Efficacy and safety of twice‐daily augmented betamethasone dipropionate lotion versus clobetasol propionate solution in patients with moderate‐to‐severe scalp psoriasis. Clinical Therapeutics 1995;17(3):390‐401. [MEDLINE: ] - PubMed
Kiss 1996 {published data only}
-
- Kiss I, McCreary HL, Siskin SB, Epinette WW. A randomized, double ‐ blind, parallel group dose ranging comparison of the efficacy and safety of calcipotriene solution in the treatment of scalp psoriasis. American Academy of Dermatology. 54th annual meeting February 10‐15. Journal of the American Academy of Dermatology 1996:P301.
Kiss 1996a {published data only}
-
- Kiss I, McCreary HL, Siskin SB, Epinette WW. A randomized, double ‐ blind, parallel group dose ranging comparison of the efficacy and safety of calcipotriene solution in the treatment of scalp psoriasis. American Academy of Dermatology. 54th annual meeting February 10‐15. Journal of the American Academy of Dermatology 1996:P301.
Klaber 1994 {published data only}
-
- Klaber MR, Hutchinson PE, Pedvis‐Leftick A, Kragballe K, Reunala TL, Kerkhof PC, et al. Comparative effects of calcipotriol solution (50 micrograms/ml) and betamethasone 17‐valerate solution (1 mg/ml) in the treatment of scalp psoriasis. British Journal of Dermatology 1994;131(5):678‐83. [MEDLINE: ] - PubMed
Klaber 2000 {published data only}
-
- Klaber MR, McKinnon C. Calcipotriol (Dovonex©) scalp solution in the treatment of scalp psoriasis: comparative efficacy with 1% coal tar/1% coconut oil/0.5% salicylic acid (Capasal©) shampoo, and long‐term experience. Journal of Dermatological Treatment 2000;11(1):21‐8. [EMBASE: 2000114933]
Köse 1997 {published data only}
-
- Köse O. Calcipotriol ointment vs clobetasol solution in scalp psoriasis. Journal of Dermatological Treatment 1997;8(4):287. [EMBASE: 1998022654]
Kragballe 2009 {published data only (unpublished sought but not used)}
-
- Kragballe K, Ganslandt C, Ortonne JP. A calcipotriol plus betamethasone dipropionate scalp formulation significantly reduces itching in scalp psoriasis. Abstract P3343. American Academy of Dermatology 67th Annual Meeting March 6‐10, 2009. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB172.
-
- Kragballe K, Hoffmann V, Ortonne JP, Tan J, Nordin P, Segaert S. Efficacy and safety of calcipotriol plus betamethasone dipropionate scalp formulation compared with calcipotriol scalp solution in the treatment of scalp psoriasis: a randomized controlled trial. British Journal of Dermatology 2009;161(1):159‐66. [MEDLINE: ] - PubMed
-
- NCT00243464. A calcipotriol plus betamethasone diproprionate scalp formulation significantly reduces itching in scalp. www.clinicaltrials.gov/show/NCT00243464?term=NCT00243464 (accessed 5 November 2014).
-
- Ortonne JP, Ganslandt C, Tan J, Nordin P, Kragballe K, Segaert S. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. Journal of the European Academy of Dermatology & Venereology 2009;23(8):919‐26. [MEDLINE: ] - PubMed
Lassus 1976 {published data only}
-
- Lassus A. Local treatment of psoriasis of the scalp with clobetasol propionate and betamethasone‐17,21‐dipropionate: a double‐blind comparison. Current Medical Research & Opinion 1976;4(5):365‐7. [MEDLINE: ] - PubMed
Lepaw 1978 {published data only}
-
- Lepaw MI. Double‐blind comparison of halcinonide solution and placebo control in treatment of psoriasis of the scalp. Cutis 1978;21(4):571‐3. [MEDLINE: ] - PubMed
Luger 2008 {published data only}
-
- Cambazard F. Xamiol, a new treatment in scalp psoriasis: short‐ and long‐term results [Un nouveau traitement dans le psoriasis du cuir chevelu : Xamiol®: Résultats à court terme et à long terme]. Annales de Dermatologie et de Vénéréologie 2009;136(Suppl 3):546‐50. [EMBASE: 2009406816]
Medansky 1974 {published data only}
-
- Medansky RS, Handler RM. Treating psoriasis of the scalp with a new corticosteroid lotion. Illinois Medical Journal 1974;145(6):503‐4. [MEDLINE: ] - PubMed
Monk 1995 {published data only}
-
- Monk BE, Mason RBS, Munro CS, Darley CR. An open and single‐blind comparative assessment of unguentum cocois compound in the treatment of psoriasis of the scalp. Journal of Dermatological Treatment 1995;6(3):159‐61. [EMBASE: 1995319726]
NCT01195831 {published data only (unpublished sought but not used)}
-
- NCT01195831. Efficacy and safety of Xamiol® gel compared to calcipotriol scalp solution in patients with scalp psoriasis. clinicaltrials.gov/show/NCT01195831 (accessed 5 November 2014).
Olsen 1991 {published data only}
-
- Olsen EA, Cram DL, Ellis CN, Hickman JG, Jacobson C, Jenkins EE, et al. A double‐blind, vehicle‐controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. Journal of the American Academy of Dermatology 1991;24(3):443‐7. [MEDLINE: ] - PubMed
Pauporte 2004 {published data only}
-
- Pauporte M, Maibach H, Lowe N, Pugliese M, Friedman DJ, Mendelsohn H, et al. Fluocinolone acetonide topical oil for scalp psoriasis. Journal of Dermatological Treatment 2004;15(6):360‐4. [MEDLINE: ] - PubMed
Regaña 2009 {published data only}
-
- Regana MS, Mirada A, Trullás C, Dilmé E. Increased efficacy and acceptability of a nonecoal tar shampoo for scalp psoriasis: Better efficacy and acceptability. 67th Annual Meeting of the American Academy of Dermatology, AAD San Francisco, CA United States. Conference Start: 20090306 Conference End: 20090310. Journal of the American Academy of Dermatology 2009;60(3 Suppl 1):AB179. [EMBASE: 70142428]
Reygagne 2002 {published data only}
-
- Reygagne P, Diaconu J, Près H, Ernst TM, Meyer KG. Efficacy and safety comparison of clobetasol proprionate shampoo, gel and vehicle in scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 2002;16(Suppl 1):283.
Reygagne 2005 {published data only}
-
- Reygagne P, Mrowietz U, Decroix J, Waard‐van der Spek FB, Acebes LO, Figueiredo A, et al. Clobetasol propionate shampoo 0.05% and calcipotriol solution 0.005%: a randomized comparison of efficacy and safety in subjects with scalp psoriasis. Journal of Dermatological Treatment 2005;16(1):31‐6. [MEDLINE: ] - PubMed
-
- Reygagne P, Mrowietz U, Decroix J, Spek W, Olmos Acebes L, et al. Four‐week efficacy and safety comparison of a new clobetasol shampoo and calcipotriol solution 0.005% in subjects with scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 2002;16(Suppl 1):285.
Ruzicka 2004 {published data only}
-
- Ruzicka T, Trompke C. Treatment of scalp psoriasis. An effective and safe tacalcitol emulsion [Behandlung der Kopfhaut‐Psoriasis: Gute Wirksamkeit und Sicherheit durch Tacalcitol‐Emulsion]. Hautarzt 2004;55(2):165‐70. [MEDLINE: ] - PubMed
Shuttleworth 1998 {published data only}
-
- Shuttleworth D, Galloway DB, Boorman GC, Donald AE. A double‐blind, placebo‐controlled study of the clinical efficacy of ciclopirox olamine (1.5%) shampoo for the control of scalp psoriasis. Journal of Dermatological Treatment 1998;9(3):163‐7. [EMBASE: 1998346163]
Sofen 2011 {published data only}
-
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Efficacy and safety results from a randomized, double‐blind, vehicle‐controlled study of clobetasol propionate spray for the treatment of moderate to severe plaque psoriasis of the scalp (Poster P3368) 68th Annual Meeting of the American Academy of Dermatology, AAD Miami, FL United States. Journal of the American Academy of Dermatology 2010;62(3 Suppl 1):AB140. [EMBASE: 70142994]
-
- Cook‐Bolden FE, Goffe BS, Hudson CP, Sofen HL. Patient satisfaction with clobetasol propionate spray, 0.05% for the management of moderate to severe plaque psoriasis of the scalp. 70th Annual Meeting of the American Academy of Dermatology San Diego, CA United States. Conference Start: 20120316 Conference End: 20120320. Journal of the American Academy of Dermatology 2012;66(4 Suppl 1):AB197. [EMBASE: 70704639]
-
- Cook‐Bolden FE, Goffe BS, Sofen HL, Colon LE, Gottschalk RW. Successful treatment of moderate to severe plaque psoriasis of the scalp with clobetasol propionate spray, 0.05% (Abstract P3347). 69th Annual Meeting of the American Academy of Dermatology New Orleans, LA United States. Conference Start: 20110204 Conference End: 20110208. Journal of the American Academy of Dermatology 2011;64(2 Suppl 1):AB157. [EMBASE: 70331905]
-
- Cook‐Bolden FE, Goffe BS, Sofen HL, Hudson CP, Colon LE, Gottschalk RW, et al. Patient satisfaction with clobetasol proprionate spray, 0.05% for the treatment of moderate to severe plaque psoriasis of the scalp. 36th Annual Hawaii Dermatology Seminar of the Skin Disease Education Foundation Waikoloa, HI United States. Conference Start: 20120219 Conference End: 20120224. Seminars in Cutaneous Medicine & Surgery 2012;31(1):A9‐A10. [EMBASE: 70706927]
-
- Sofen H, Hudson CP, Cook‐Bolden FE, Preston N, Colon LE, Caveney SW, et al. Clobetasol propionate 0.05% spray for the management of moderate‐to‐severe plaque psoriasis of the scalp: results from a randomized controlled trial. Journal of Drugs in Dermatology 2011;10(8):885‐92. [MEDLINE: ] - PubMed
Swinehart 1989 {published data only}
-
- Swinehart JM, Barkoff JR, Dvorkin D, Fisher G, Peets E. Mometasone furoate lotion once daily versus triamcinolone acetonide lotion twice daily in psoriasis. International Journal of Dermatology 1989;28(10):680‐1. [MEDLINE: ] - PubMed
Tyring 2010 {published data only}
-
- Tyring S, Mendoza N, Appell M, Bibby A, Foster R, Hamilton T, et al. A calcipotriene/betamethasone dipropionate two‐compound scalp formulation in the treatment of scalp psoriasis in Hispanic/Latino and Black/African American patients: results of the randomized, 8‐week, double‐blind phase of a clinical trial. International Journal of Dermatology 2010;49(11):1328‐33. [MEDLINE: ] - PubMed
van de Kerkhof 2002 {published data only}
-
- Kerkhof PC, Green C, Hamberg KJ, Hutchinson PE, Jensen JK, Kidson P, et al. Safety and efficacy of combined high‐dose treatment with calcipotriol ointment and solution in patients with psoriasis. Dermatology 2002;204(3):214‐21. [MEDLINE: ] - PubMed
van de Kerkhof 2009 {published data only}
-
- Kerkhof P, Anstey A, Barnes L, Bolduc C. A new scalp formulation of calcipotriene plus betamethasone in the treatment of scalp psoriasis compared to its active ingredients in the same vehicle. Journal of the American Academy of Dermatology 2007;56(2):AB182.
-
- Kerkhof PC, Hoffmann V, Anstey A, Barnes L, Bolduc C, Reich K, et al. A new scalp formulation of calcipotriol plus betamethasone dipropionate compared with each of its active ingredients in the same vehicle for the treatment of scalp psoriasis: a randomized, double‐blind, controlled trial. British Journal of Dermatology 2009;160(1):170‐6. [MEDLINE: ] - PubMed
Van der Ploeg 1989 {published data only}
-
- Ploeg DE, Cornell RC, Binder R, Weintraub JS, Jarratt M, Jones ML, et al. Clinical trial in scalp psoriasis mometasone furoate lotion 0.1% applied once daily vs betamethasone valerate lotion 0.1% applied twice daily. Acta Therapeutica 1989;15(2):145‐52.
Wall 1999 {published data only}
-
- Wall ARJ, Bibby AJ. Combined use of calcipotriol solution (50 ug/ml) and Polytar Liquid in scalp psoriasis (Abstract P‐689). Journal of the European Academy of Dermatology & Venereology 1999;12(Suppl 2):S337.
Wilhelm 2013 {published data only (unpublished sought but not used)}
-
- Wilhem KP, Ocker G, Tebbs V, Guilabert A. A new mometasone formulation (LAS 41002 emulsion with 36% water content) is effective, well tolerated and cosmetically acceptable in the treatment of scalp psoriasis. Abstract ‐ 22nd Congress of the European Academy of Dermatology and Venereology, 2‐6 October 2013, Istanbul. Journal of the European Academy of Dermatology & Venereology. 2013.
Willis 1986 {published data only}
-
- Willis I, Cornell RC, Penneys NS, Zaias N. Multicenter study comparing 0.05% gel formulations of desoximetasone and fluocinonide in patients with scalp psoriasis. Clinical Therapeutics 1986;8(3):275‐82. [MEDLINE: ] - PubMed
Wright 1985 {published data only}
-
- Wright S, Mann RJ. Comparison of a cream containing 0.1% dithranol in a 17% urea base (Psoradrate(TM)) with coal tar pomade in the treatment of scalp psoriasis. Clinical & Experimental Dermatology 1985;10(4):375‐8. [MEDLINE: ] - PubMed
Yilmaz 2005 {published data only}
-
- Yilmaz H, Aydin F, Senturk N, Canturk T, Turanli AY. A comparison between the effects of calcipotriol lotion, momethasone furoate lotion and their combinations for the treatment of scalp psoriasis [Kalsipotriol Losyon, Mometazon Furoat Losyonve Kombinasyonlarinin Saçli Deri Psoriazisi Tedavisindeki Etkinliginin Karsilastirilmasi]. Turkderm Deri Hastaliklari ve Frengi Arsivi 2005;39(2):109‐14. [EMBASE: 2006204571]
References to studies excluded from this review
Andreassi 2003 {published data only}
-
- Andreassi L, Giannetti A, Milani M, Scale Investigators Group. Efficacy of betamethasone valerate mousse in comparison with standard therapies on scalp psoriasis: an open, multicentre, randomized, controlled, cross‐over study on 241 patients. British Journal of Dermatology 2003;148(1):134‐8. [MEDLINE: ] - PubMed
-
- Milani M. Efficacy of bettamousse on scalp psoriasis: the scale trial. Annales de Dermatologie et de Vénéréologie 2002;129:P2007.
Bohnsack 2004 {published data only}
-
- Bohnsack K, Upmeyer H, Albrecht H, Treder‐Conrad C, Scholermann A, Rippke F, et al. In‐vivo assessment of the efficacy of an innovative anti‐dandruff shampoo in subjects with scalp dermatitis (Abstract P02.1). 2004 Forum for Nordic Dermato‐Venereology;9(Suppl 7):43.
Cassano 2007 {published data only}
-
- Cassano N, Vena GA. Treatment of scalp psoriasis with betamethasone dipropionate and calcipotriol two‐compound product. Acta Dermato‐Venereologica 2007;87(1):85‐6. [MEDLINE: ] - PubMed
Cunliffe 1974 {published data only}
-
- Cunliffe WJ, Dodman B. A comparison of tar liquid and cetrimide shampoo in the management of psoriasis of the scalp. British Journal of Clinical Practice 1974;28(9):314‐5. [MEDLINE: ] - PubMed
Elie 1983 {published data only}
-
- Elie R, Durocher LP, Kavalec EC. Effect of salicylic acid on the activity of betamethasone‐17,21‐dipropionate in the treatment of erythematous squamous dermatoses. Journal of International Medical Research 1983;11(2):108‐12. [MEDLINE: ] - PubMed
Fallica 1989 {published data only}
-
- Fallica L, Prima T, Gasparri O, Panebianco R, Benedetti M. Clobetasone 17‐butyrate, lotion scalp fluid. Results of a clinical trial in the treatment of steroid‐sensitive dermatitis. Clinical Trials Journal 1989;26(6):393‐400. [EMBASE: 1990069772]
Feng 1997 {published data only}
-
- Feng JC, Wang JB, Yu BT, Sun QN. Coal tar lotion in the treatment of 31 cases of scalp psoriasis. Chinese Journal of Dermatology 1997;30(2):84.
Heydendael 2004 {published data only}
-
- Heydendael VMR, Borgie CAJM, Spuls PI, Bossuyt PMM, Bos JD, Rie MA. The burden of psoriasis is not determined by disease severity only. Journal of Investigative Dermatology Symposium Proceedings 2004;9(2):131‐5. [EMBASE: 2004161603] - PubMed
Jakubowicz 1981 {published data only}
-
- Jakubowicz K, Ochman U. Psoriazin ointment in the treatment of psoriasis of the scalp (preliminary results) [Zastosowane masci psoriazin w leczeniu luszczycy skory owlosionej glowy (wyniki wstepne)]. Przeglad Dermatologiczny 1981;68(1):95‐9. [MEDLINE: ] - PubMed
Kar 2000 {published data only}
-
- Kar PK, Rama Sastry CV. A study of combination therapy with puvasol and topical corticosteroid in psoriasis vulgaris involving scalp. Indian Journal of Dermatology 2000;43(3):130‐5.
Kose 1995 {published data only}
-
- Kose O. 5 and 10 days occlusive treatment with calcipotriol in scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 1995;3(Suppl 1):S148.
Kostarelos 2000 {published data only}
-
- Kostarelos K, Teknetzis A, Lefaki I, Ioannides D, Minas A. Double‐blind clinical study reveals synergistic action between alpha‐hydroxy acid and betamethasone lotions towards topical treatment of scalp psoriasis. Journal of the European Academy of Dermatology & Venereology 2000;14(1):5‐9. [EMBASE: 2001338533] - PubMed
Lassus 1985 {published data only}
-
- Lassus A, Lauharanta J, Kanerva L, Juvakoski T. Treatment of scalp psoriasis. A new dexamethasone preparation in a double‐blind trial with hydrocortisone‐17‐butyrate [Behandlung der Kopfhautpsoriasis: Ein neues Dexamethason‐Präparat im Doppelblindversuch mit Hydrocortison‐17‐butyrat]. Schweizerische Rundschau für Medizin/Praxis 1985;74(15):382‐3. [EMBASE: 1985111490] - PubMed
Lassus 1991 {published data only}
-
- Lassus A, Forsstrom S. A double‐blind study comparing oleum horwathiensis with placebo in the treatment of psoriasis. Journal of International Medical Research 1991;19(2):137‐46. [EMBASE: 1991173914] - PubMed
Lecewicz‐Torun 2001 {published data only}
-
- Lecewicz‐Torun B, Chodorowska G. Clinical evaluation of hair shampoo 'Zdroj' for therapeutic use [Ocena kliniczna szamponu leczniczego do mycia wlosow 'Zdroj']. Przeglad Dermatologiczny 2001;88(2):177‐80. [EMBASE: 2001227779]
Liu 1994 {published data only}
-
- Liu JH, Sun JF, Zeng XS, Zhang Q, Lin ML. Clinical observation on the efficacy of halometasone/triclosan (Sicorten Plus) cream in the treatment of scalp psoriasis. Chinese Journal of Dermatology 1994;27(5):325.
Nolting 1983 {published data only}
-
- Nolting S, Hagemeier HH. Betamethasone‐17,21‐dipropionate plus salicylic acid compared with betamethasone‐dipropionate solution in the therapy of erythemato‐squamous dermatoses [Therapie erythrosquamöser Dermatosen. Betamethason‐Diproprionat plus Salizylsäure im Vergleich zu Betamethason‐Diproprionat‐Losung]. Fortschritte der Medizin 1983;101(37):1679‐83. [EMBASE: 1983237084] - PubMed
Rex 1973 {published data only}
-
- Rex Jr IH. A controlled evaluation of a new topical corticosteroid in psoriasis. Current Therapeutic Research ‐ Clinical and Experimental 1973;15(11):833‐8. [EMBASE: 0974140513] - PubMed
Ross 1981 {published data only}
-
- Ross SD, Schachter RK. A randomized comparison of three conventional modes of treatment of psoriasis of the scalp. Cutis 1981;28(4):438‐9. [EMBASE: 1982071447] - PubMed
Saraceno 2014 {published data only}
-
- Saraceno R, Camplone G, D'Agostino M, Simone C, Cesare A, Filosa G, et al. Efficacy and maintenance strategies of two‐compound formulation calcipotriol and betamethasone dipropionate gel (Xamiol gel) in the treatment of scalp psoriasis: results from a study in 885 patients. Journal of Dermatological Treatment 2014;25(1):30‐3. [EMBASE: 2013799031] - PubMed
Singh 2013 {published data only}
-
- Singh S, Byadgi PS, Rai NP. Clinical evaluation of Virechan therapy and Haridradi Vati and oil for the management of Kitibh Kushtha (psoriasis). International Journal of Research in Ayurveda and Pharmacy 2013;4(2):207‐11. [EMBASE: 2013400892]
Taneja 2004 {published data only}
-
- Taneja A, Racette A, Gourgouliatos Z, Taylor CR. Broad‐band UVB fiber‐optic comb for the treatment of scalp psoriasis: a pilot study. International Journal of Dermatology 2004;43(6):462‐7. [EMBASE: 2004286060] - PubMed
Texier 1978 {published data only}
-
- Texier L, Gauthier O. Clinical trial of a lotion containing betamethasone dipropionate in the treatment of scalp dermatoses [Experimentation clinique d'une lotion au dipropionate de betamethasone (diprosone) dans le traitement des dermatoses du cuir chevelu]. Bordeaux Medical 1978;11(3):231‐5. [EMBASE: 0978268779]
Tsankov 1995 {published data only}
-
- Tsankov N. Treatment of scalp psoriasis with 2% ketoconazole shampoo and Psanol®. Journal of the European Academy of Dermatology & Venereology 1995;5(Suppl 1):S104.
Tsankov 1998 {published data only}
-
- Tsankov N. Treatment of scalp psoriasis with Psanol and 2% ketaconazol shampoo: a double‐blind, placebo‐controlled study. Journal of the European Academy of Dermatology & Venereology 1998;11(Suppl 2):S287.
Williams 1967 {published data only}
Wulff‐Woesten 2004 {published data only}
-
- Wulff‐Woesten A, Ohlendorf D, Henz BM, Haas N. Dithranol in an emulsifying oil base (bio‐wash‐oil) for the treatment of psoriasis of the scalp. Skin Pharmacology and Physiology 2004;17(2):91‐7. [EMBASE: 2004093096] - PubMed
References to studies awaiting assessment
Andres 2005 {published data only}
-
- Andres P. Safety assessment of clobetasol proprionate 0.05% shampoo: hypothalamic‐pituitary‐adrenal axis suppression, atrophogenicity, and ocular safety in subjects with psoriasis of the scalp. 7th Asian Congress of Dermatology incorporating the 5th Regional Conference of Paediatric Dermatology Kuala Lumpur, Malaysia 28th September ‐ 1st October 2005. 2005:369. [CENTRAL: CN‐00602630]
Augustin 2014 {published data only (unpublished sought but not used)}
-
- NCT01914627. Parallel group trial to evaluate the efficacy and safety of Loion® compared to 10% salicylic acid in patients with chronic psoriasis capitis. www.clinicaltrials.gov/show/NCT01914627 (accessed 29 April 2015).
Berth‐Jones 1998 {published data only}
-
- Berth‐Jones J. A multi‐centre double blind randomised placebo‐controlled parallel group comparison to investigate the efficacy and safety of tacalcitol lotion in patients with psoriasis of the scalp. Dermatology Department, Walsgrave Hospitals NHS Trust, Clifford 1998.
Bewley 2001 {published data only}
-
- Bewley AP. The efficacy and safety of clobetasol proprionate 0.05% shampoo compared to Polytar Liquid in the treatment of scalp psoriasis. Whipps Cross University Hospital NHS Trust 2001.
Combemale 2009 {published data only}
-
- Combemale P. A new treatment in scalp psoriasis: Xamiol formulation and results at 8 weeks [Un nouveau traitement dans le psoriasis du cuir chevelu: Carte d'identite Xamiol et resultats a 8 semaines]. Annales de Dermatologie et de Vénéréologie 2009;136(Suppl 3):542‐6. [EMBASE: 2009406815]
Graham‐Brown 2001 {published data only}
-
- Graham‐Brown R. The efficacy and safety of Clobetasol proprionate 0.5% shampoo compared to Polytar Liquid in the treatment of scalp psoriasis. (Protocol RD.03.SPR.2648). University Hospitals of Leicester, UK 2001.
Groves 2001 {published data only}
-
- Groves RW. The efficacy and safety of Agent A 0.05% shampoo compared to Agent B liquid in the treatment of scalp psoriasis. Dermatology, Dermatology Department, The Middlesex Hospital, Mortimer 2001.
Hutchinson 1995 {published data only}
-
- Hutchinson P. Calcitriol solution versus Capasal in scalp psoriasis. University Hospitals of Leicester NHS Trust 1995.
Hutchinson 1997 {published data only}
-
- Hutchinson P. A multicentre, double blind, randomised, placebo controlled parallel group comparison to investigate the efficacy and safety of tacalcitol lotion (4µg/g) in patients with psoriasis of the scalp. University Hospitals of Leicester NHS Trust 1997.
Lebwohl 2015 {published data only}
-
- Lebwohl M, Qureshi A, Kianifard F, Steadman J, Guana A, Nyirady J. Secukinumab in the treatment of moderate to severe scalp psoriasis: a study to evaluate efficacy and safety. 73rd Annual Meeting of the American Academy of Dermatology San Francisco, CA United States. Conference Start: 2015/03/20 Conference End: 2015/03/24. Journal of the American Academy of Dermatology 2015;72(5 Suppl 1):AB250.
Messenger 2011 {published data only}
-
- Messenger A. Multi‐centre, randomised, active controlled, investigator blinded, parallel comparison study of the efficacy and safety of clobetasol proprionate 0.05% shampoo compared to Polytar Liquid® in the treatment of scalp psoriasis. Dermatology, Directorate of Medicine, Royal Hallamshire, Central Sheffield University Hospitals NHS Trust 2001.
Nishiyama 2010 {published data only}
-
- Nishiyama H, Zenke Y, Kajiwara T, Kobayashi R, Nakano T, Harada H, et al. The usefulness of camellia oil spray and camellia oil‐containing shampoo for treatment of scaling and itching in patients with psoriasis vulgaris. Nishinihon Journal of Dermatology 2010;72(4):405‐13. [EMBASE: 2010483819]
Pye 1995 {published data only}
-
- Pye RJ. MC392 ‐ Efficacy and safety of calcipotriol cream in the treatment of scalp psoriasis. Box No 46, Addenbrooke's NHS Trust, CB2 2QQ 1995.
Pye 1997 {published data only}
-
- Pye RJ. Calcipotriol solution versus Capasal shampoo in scalp psoriasis. Long term treatment with calcipotriol scalp solution (50µg/ml) in patients with scalp psoriasis. Box No 46, Addenbrooke's NHS Trust, CB2 2QQ 1997.
References to ongoing studies
EUCTR2010‐024033‐24‐DE {published data only}
-
- 2010‐024033‐24. Double blind, randomized, clinical study to compare the efficacy and safety of betamethasone 0,05% salicylic acid 2% vs. Diprosalic solution vs. vehicle for the treatment of psoriasis capitis [Doppelblinde, randomisierte klinische Studie zum Vergleich der Wirksamkeit und Verträglichkeit von Betamethason 0,05% Salicylsäure 2% Lösung vs. Diprosalic Lösung vs. Grundlage bei Patienten mit Psoriasis der Kopfhaut]. www.clinicaltrialsregister.eu/ctr‐search/search?query=2010‐024033‐24 (accessed 20 November 2014).
NCT01368887 {published data only}
-
- NCT01368887. Study to test the effectiveness of a new treatment for scalp psoriasis. www.clinicaltrials.gov/show/NCT01368887 (accessed 28 April 2015).
NCT01582932 {published data only}
-
- NCT01582932. To evaluate the safety, tolerability and efficacy of calcipotriene foam, 0.005%, versus vehicle foam in pediatric subjects (ages 2‐11) with plaque psoriasis. www.clinicaltrials.gov/show/NCT01582932 (accessed 20 November 2014).
NCT01707043 {published data only}
-
- NCT01707043. Patient preference of Taclonex ointment to Taclonex scalp suspension in adult subjects with psoriasis vulgaris (PS Taclonex). https://www.clinicaltrials.gov/ct2/show/record/NCT01707043 (accessed 28 September 2015).
NCT02413229 {published data only}
-
- * NCT02413229. A randomized double blind vehicle controlled dose ranging parallel design multiple site clinical study. https://www.clinicaltrials.gov/ct2/show/record/NCT02413229 (accessed 28 September 2015).
NCT02533973 {published data only}
-
- * NCT02533973. Long‐term treatment of scalp psoriasis with Xamiol® gel in a large adult Chinese population. https://www.clinicaltrials.gov/ct2/show/record/NCT02533973 (accessed 28 September 2015).
Additional references
Abel 1986
-
- Abel EA, DiCicco LM, Orenberg EK, Fraki JE, Farber EM. Drugs in exacerbation of psoriasis. Journal of the American Academy of Dermatology 1986;15(5 Pt 1):1007‐22. [MEDLINE: ] - PubMed
Al'Abadie 1994
-
- Al'Abadie MS, Kent GG, Gawkrodger DJ. The relationship between stress and the onset and exacerbation of psoriasis and other skin conditions. British Journal of Dermatology 1994;130(2):199‐203. [MEDLINE: ] - PubMed
Arnold 1997
-
- Arnold WP. Tar. Clinics in Dermatology 1997;15(5):739‐44. [MEDLINE: ] - PubMed
Austin 1999
-
- Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon‐gamma, interleukin‐2, and tumor necrosis factor‐alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. Journal of Investigative Dermatology 1999;113(5):752‐9. [MEDLINE: ] - PubMed
Barker 1991
-
- Barker JN. The pathophysiology of psoriasis. Lancet 1991;338(8761):227‐30. [MEDLINE: ] - PubMed
Baroni 2004
-
- Baroni A, Paoletti I, Ruocco E, Agozzino M, Tufano MA, Donnarumma G. Possible role of Malassezia furfur in psoriasis: modulation of TGF‐beta1, integrin, and HSP70 expression in human keratinocytes and in the skin of psoriasis‐affected patients. Journal of Cutaneous Pathology 2004;31(1):35‐42. [MEDLINE: ] - PubMed
Bata‐Csorgo 1995
-
- Bata‐Csorgo Z, Hammerberg C, Voorhees JJ, Cooper KD. Intralesional T‐lymphocyte activation as a mediator of psoriatic epidermal hyperplasia. Journal of Investigative Dermatology 1995;105(1 Suppl):89S‐94S. [MEDLINE: ] - PubMed
Bos 2008
-
- Bos JD, Spuls PI. Topical treatments in psoriasis: today and tomorrow. Clinics in Dermatology 2008;26(5):432‐7. [MEDLINE: ] - PubMed
Chan 2009
-
- Chan CS, Voorhees AS, Lebwohl MG, Korman NJ, Young M, Bebo BF Jr, et al. Treatment of severe scalp psoriasis: from the Medical Board of the National Psoriasis Foundation. Journal of the American Academy of Dermatology 2009;60(6):962‐71. [MEDLINE: ] - PubMed
Chang 2009
-
- Chang YT, Chen TJ, Liu PC, Chen YC, Chen YJ, Huang YL, et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Dermato‐Venereologica 2009;89(3):262‐6. [MEDLINE: ] - PubMed
Chaput 1985
Christensen 2006
-
- Christensen PM, Kristiansen IS. Number‐needed‐to‐treat (NNT)‐‐needs treatment with care. Basic & Clinical Pharmacology & Toxicology 2006;99(1):12‐6. [MEDLINE: ] - PubMed
Cosmetic Ingredient Review Expert Panel 2008
-
- Cosmetic Ingredient Review Expert Panel. Final safety assessment of coal tar as used in cosmetics. International Journal of Toxicology 2008;27(Suppl 2):1‐24. [PUBMED: 18830861] - PubMed
Day 2008
-
- Day I, Lin AN. Use of pimecrolimus cream in disorders other than atopic dermatitis. Journal of Cutaneous Medicine & Surgery 2008;12(1):17‐26. [MEDLINE: ] - PubMed
Del Rosso 2011
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [MEDLINE: ] - PubMed
Devrimci‐Ozguven 2000
-
- Devrimci‐Ozguven H, Kundakci TN, Kumbasar H, Boyvat A. The depression, anxiety, life satisfaction and affective expression levels in psoriasis patients. Journal of the European Academy of Dermatology & Venereology 2000;14(4):267‐71. [MEDLINE: ] - PubMed
Dika 2007
-
- Dika E, Bardazzi F, Balestri R, Maibach HI. Environmental factors and psoriasis. Current Problems in Dermatology 2007;35:118‐35. [PUBMED: 17641494] - PubMed
Dogra 2010
-
- Dogra S, Kaur I. Childhood psoriasis. Indian Journal of Dermatology, Venereology & Leprology 2010;76(4):357‐65. [MEDLINE: ] - PubMed
Dowlatshahi 2014
-
- Dowlatshahi EA, Wakkee M, Arends LR, Nijsten T. The prevalence and odds of depressive symptoms and clinical depression in psoriasis patients: a systematic review and meta‐analysis. Journal of Investigative Dermatology 2014;134(6):1542‐51. [MEDLINE: ] - PubMed
EMA 2004
-
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medical products indicated for the treatment of psoriasis, 18 November 2004. www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/... (accessed 20 November 2014).
Faergemann 2007
-
- Faergemann J, Borgers M, Degreef H. A new ketoconazole topical gel formulation in seborrhoeic dermatitis: an updated review of the mechanism. Expert Opinion on Pharmacotherapy 2007;8(9):1365‐71. [MEDLINE: ] - PubMed
Fluhr 2008
-
- Fluhr J, Cavallotti C, Berardesca E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clinics in Dermatology 2008;26(4):380‐6. [MEDLINE: ] - PubMed
Frankel 2010
Frez 2014
-
- Frez ML, Asawanonda P, Gunasekara C, Koh C, Loo S, Oon HH, et al. Recommendations for a patient‐centred approach to the assessment and treatment of scalp psoriasis: a consensus statement from the Asia Scalp Psoriasis Study Group. Journal of Dermatological Treatment 2014; Vol. 25, issue 1:38‐45. - PubMed
Gardinal 2009
-
- Gardinal I, Ammoury A, Paul C. Moderate to severe psoriasis: from topical to biological treatment. Journal of the European Academy of Dermatology & Venereology 2009;23(11):1324‐6. [PUBMED: 19470061] - PubMed
Ginsburg 1993
-
- Ginsburg IH, Link BG. Psychosocial consequences of rejection and stigma feelings in psoriasis patients. International Journal of Dermatology 1993;32(8):587‐91. [MEDLINE: ] - PubMed
Gomez‐Moyano 2014
-
- Gomez‐Moyano E, Crespo‐Erchiga V, Martinez‐Pilar L, Godoy Diaz D, Martinez‐Garcia S, Lova Navarro M, et al. Do Malassezia species play a role in exacerbation of scalp psoriasis? [Les levures Malassezia jouent‐elles un rôle dans les poussées du psoriasis du cuir chevelu?]. Journal de Mycologie Médicale 2014;24(2):87‐92. [MEDLINE: ] - PubMed
Griffiths 2007
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263‐71. [MEDLINE: ] - PubMed
Gudjonsson 2008
-
- Gudjonsson JE. Genetic variation and psoriasis. Giornale Italiano di Dermatologia e Venereologia 2008;143(5):299‐305. [MEDLINE: ] - PubMed
Hegemann 1992
-
- Hegemann L, Fruchtmann R, Rooijen LA, Muller‐Peddinghaus R, Mahrle G. The antipsoriatic drug, anthralin, inhibits protein kinase C‐‐implications for its mechanism of action. Archives of Dermatological Research 1992;284(3):179‐83. [MEDLINE: ] - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horn 2010
-
- Horn EJ, Domm S, Katz HI, Lebwohl M, Mrowietz U, Kragballe K, et al. Topical corticosteroids in psoriasis: strategies for improving safety. Journal of the European Academy of Dermatology & Venereology 2010;24(2):119‐24. [MEDLINE: ] - PubMed
Jales 2012
Khan 1981
-
- Khan SA, Williamson DM, Gatecliff M, Tosh J. The treatment of chronic psoriasis a two‐centre comparative study. Practitioner 1981;225(1356):932‐4. [MEDLINE: ] - PubMed
Kim 2011
-
- Kim GW, Jung HJ, Ko HC, Kim MB, Lee WJ, Lee SJ, et al. Dermoscopy can be useful in differentiating scalp psoriasis from seborrhoeic dermatitis. British Journal of Dermatology 2011;164(3):652‐6. [MEDLINE: ] - PubMed
Kragballe 1990
-
- Kragballe K, Wildfang IL. Calcipotriol (MC 903), a novel vitamin D3 analogue stimulates terminal differentiation and inhibits proliferation of cultured human keratinocytes. Archives of Dermatological Research 1990;282(3):164‐7. [MEDLINE: ] - PubMed
Kragballe 1993
-
- Kragballe K, Iversen L. Calcipotriol. A new topical antipsoriatic. Dermatologic Clinics 1993;11(1):137‐41. [MEDLINE: ] - PubMed
Krueger 1984
-
- Krueger GG, Bergstresser PR, Lowe NJ, Voorhees JJ, Weinstein GD. Psoriasis. Journal of the American Academy of Dermatology 1984;11(5 Pt 2):937‐47. [MEDLINE: ] - PubMed
Li 2012
Luger 2007
-
- Luger T, Paul C. Potential new indications of topical calcineurin inhibitors. Dermatology: International Journal for Clinical and Investigative Dermatology 2007;215(Suppl 1):45‐54. [MEDLINE: ] - PubMed
Mahrle 1994
-
- Mahrle G, Bonnekoh B, Wevers A, Hegemann L. Anthralin: how does it act and are there more favourable derivatives?. Acta Dermato‐Venereologica. Supplementum. 1994;186:83‐4. [MEDLINE: ] - PubMed
Mashaly 2011
-
- Mashaly HM, Masood NA, Mohamed AS. Classification of papulo‐squamous skin diseases using image analysis. Skin Research & Technology 2012;18(1):36‐44. [MEDLINE: ] - PubMed
Mason 2013
-
- Mason AR, Mason JM, Cork MJ, Hancock H, Dooley G. Topical treatments for chronic plaque psoriasis of the scalp: a systematic review. British Journal of Dermatology 2013;169(3):519‐27. [MEDLINE: ] - PubMed
Menter 2010
-
- Menter A. Goeckerman therapy versus biologics in the treatment of psoriasis. Journal of the American Academy of Dermatology 2010;62(3):516‐7. [MEDLINE: ] - PubMed
Niedner 1996
-
- Niedner R. Glucocorticosteroids in dermatology [Glukokortikosteroide in der Dermatologie]. Dt Ärzteblatt 1996;93(44):A2868‐72. [www.aerzteblatt.de/pdf/93/44/a2868_2.pdf]
Niwa 2003
-
- Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. British Journal of Dermatology 2003;149(5):960‐7. [MEDLINE: ] - PubMed
Ortonne 2009
-
- Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trueb RM. Scalp psoriasis: European consensus on grading and treatment algorithm. Journal of the European Academy of Dermatology & Venereology 2009;23(12):1435‐44. [MEDLINE: ] - PubMed
Paghdal 2009
-
- Paghdal KV, Schwartz RA. Topical tar: back to the future. Journal of the American Academy of Dermatology 2009;61(2):294‐302. [MEDLINE: ] - PubMed
Panhans‐Gross 2001
-
- Panhans‐Gross A, Novak N, Kraft S, Bieber T. Human epidermal Langerhans' cells are targets for the immunosuppressive macrolide tacrolimus (FK506). Journal of Allergy & Clinical Immunology 2001;107(2):345‐52. [MEDLINE: ] - PubMed
Papp 2007
-
- Papp K, Berth‐Jones J, Kragballe K, Wozel G, Brassinne M. Scalp psoriasis: a review of current topical treatment options. Journal of the European Academy of Dermatology & Venereology 2007;21(9):1151‐60. [MEDLINE: ] - PubMed
Patel 2008
-
- Patel V, Horn EJ, Lobosco SJ, Fox KM, Stevens SR, Lebwohl M. Psoriasis treatment patterns: results of a cross‐sectional survey of dermatologists. Journal of the American Academy of Dermatology 2008;58(6):964‐9. [MEDLINE: ] - PubMed
Prinz 1994
-
- Prinz JC, Gross B, Vollmer S, Trommler P, Strobel I, Meurer M, et al. T cell clones from psoriasis skin lesions can promote keratinocyte proliferation in vitro via secreted products. European Journal of Immunology 1994;24(3):593‐8. [MEDLINE: ] - PubMed
Puig 2010
-
- Puig L, Ribera M, Hernanz JM, Belinchon I, Santos‐Juanes J, Linares M, et al. Treatment of scalp psoriasis: review of the evidence and Delphi consensus of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology [Tratamiento de la psoriasis del cuero cabelludo. Revisión de la evidencia y Consenso Delphi del Grupo de Psoriasis de la Academia Española de Dermatología y Venereología]. Actas Dermo‐Sifiliográficas 2010;101(10):827‐46. [MEDLINE: ] - PubMed
Rapp 1999
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401‐7. [MEDLINE: ] - PubMed
Reich 2009
-
- Reich K, Krüger K, Mössner R, Augustin M. Epidemiology and clinical pattern of psoriatic arthritis in Germany: a prospective interdisciplinary epidemiological study of 1511 patients with plaque‐type psoriasis. British Journal of Dermatology 2009;160(5):1040‐7. [MEDLINE: ] - PubMed
Roques 2006
-
- Roques C, Brousse S, Panizzutti C. In vitro antifungal efficacy of ciclopirox olamine alone and associated with zinc pyrithione compared to ketoconazole against Malassezia globosa and Malassezia restricta reference strains. Mycopathologia 2006;162(6):395‐400. [MEDLINE: ] - PubMed
Rosenberg 1982
-
- Rosenberg EW, Belew PW. Improvement of psoriasis of the scalp with ketoconazole. Archives of Dermatology 1982;118(6):370‐1. [MEDLINE: ] - PubMed
Rzany 1998
-
- Rzany B, Naldi L, Schafer T, Stern R, Williams H. The diagnosis of psoriasis: diagnostic criteria. British Journal of Dermatology 1998;138(5):917. [MEDLINE: ] - PubMed
Samarasekera 2013
-
- Samarasekera EJ, Sawyer L, Wonderling D, Tucker R, Smith CH. Topical therapies for the treatment of plaque psoriasis: systematic review and network meta‐analyses. British Journal of Dermatology 2013;168(5):954‐67. [MEDLINE: ] - PubMed
Setty 2007
Shokeen 2014
-
- Shokeen J, O'Neill JL, Taheri A, Feldman SR. Are topical keratolytic agents needed in the treatment of scalp psoriasis?. Dermatology Online Journal 2014;20(3):‐. [MEDLINE: ] - PubMed
Shuster 1972
-
- Shuster S. Psoriatic alopecia. British Journal of Dermatology 1972;87(1):73‐7. [MEDLINE: ] - PubMed
Staubach 2014
-
- Staubach P, Lunter DJ. Basic or maintenance therapy in dermatology. Appropriate vehicles, possibilities and limitations [Basistherapie in der Dermatologie. Geeignete Grundlagen, Möglichkeiten und Grenzen]. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 2014;65(1):63‐72; quiz 73‐4. [MEDLINE: ] - PubMed
Stern 1997
-
- Stern RS. Psoriasis. Lancet 1997;350(9074):349‐53. [MEDLINE: ] - PubMed
Tagami 1997
-
- Tagami H. Triggering factors. Clinics in Dermatology 1997;15(5):677‐85. [MEDLINE: ] - PubMed
Telfer 1992
-
- Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Archives of Dermatology 1992;128(1):39‐42. [MEDLINE: ] - PubMed
Tobin 2009
-
- Tobin AM, Higgins EM, Norris S, Kirby B. Prevalence of psoriasis in patients with alcoholic liver disease. Clinical & Experimental Dermatology 2009;34(6):698‐701. [MEDLINE: ] - PubMed
Trowbridge 2014
-
- Trowbridge RM, Pittelkow MR. Epigenetics in the pathogenesis and pathophysiology of psoriasis vulgaris. Journal of Drugs in Dermatology 2014;13(2):111‐8. [MEDLINE: ] - PubMed
Tsoi 2012
Valdimarsson 1995
-
- Valdimarsson H, Baker BS, Jonsdottir I, Powles A, Fry L. Psoriasis: a T‐cell‐mediated autoimmune disease induced by streptococcal superantigens?. Immunology Today 1995;16(3):145‐9. [MEDLINE: ] - PubMed
van de Kerkhof 1992
-
- Kerkhof PC, Chang A. Scarring alopecia and psoriasis. British Journal of Dermatology 1992;126(5):524‐5. [MEDLINE: ] - PubMed
van de Kerkhof 1998
-
- Kerkhof PC, Steegers‐Theunissen RP, Kuipers MV. Evaluation of topical drug treatment in psoriasis. Dermatology 1998;197(1):31‐6. [MEDLINE: ] - PubMed
van de Kerkhof 1998a
-
- Kerkhof PC, Hoop D, Korte J, Kuipers MV. Scalp psoriasis, clinical presentations and therapeutic management. Dermatology 1998;197(4):326‐34. [MEDLINE: ] - PubMed
van de Kerkhof 2001
-
- Kerkhof PC, Franssen ME. Psoriasis of the scalp. Diagnosis and management. American Journal of Clinical Dermatology 2001;2(3):159‐65. [MEDLINE: ] - PubMed
van de Kerkhof 2005
-
- Kerkhof PC, Kragballe K. Recommendations for the topical treatment of psoriasis. Journal of the European Academy of Dermatology & Venereology 2005;19(4):495‐9. [MEDLINE: ] - PubMed
von Stebut 2014
-
- Stebut E. Significance of topical therapy in clinical situations. Location‐dependent principles [Stellenwert der topischen Therapie in klinischen Behandlungssituationen]. Hautarzt 2014;65(3):186‐91. [MEDLINE: ] - PubMed
Weischer 2007
-
- Weischer M, Rocken M, Berneburg M. Calcineurin inhibitors and rapamycin: cancer protection or promotion?. Experimental Dermatology 2007;16(5):385‐93. [MEDLINE: ] - PubMed
Yamamoto 2000
-
- Yamamoto T, Matsuuchi M, Irimajiri J, Otoyama K, Nishioka K. Topical anthralin for psoriasis vulgaris: evaluation of 70 Japanese patients. Journal of Dermatology 2000;27(7):482‐5. [MEDLINE: ] - PubMed
Yip 1984
-
- Yip SY. The prevalence of psoriasis in the Mongoloid race. Journal of the American Academy of Dermatology 1984;10(6):965‐8. [MEDLINE: ] - PubMed
Zeljko‐Penavic 2010
-
- Zeljko‐Penavic J, Situm M, Simic D, Vurnek‐Zivkovic M. Quality of life in psoriatic patients and the relationship between type I and type II psoriasis. Collegium Antropologicum 2010;34(Suppl 1):195‐8. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

