Long-term exercise-specific neuroprotection in spinal muscular atrophy-like mice
- PMID: 26915343
- PMCID: PMC4818605
- DOI: 10.1113/JP271361
Long-term exercise-specific neuroprotection in spinal muscular atrophy-like mice
Abstract
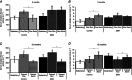
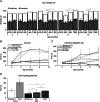
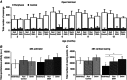
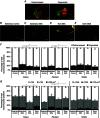
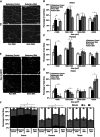
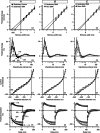
Key points: The real impact of physical exercise parameters, i.e. intensity, type of contraction and solicited energetic metabolism, on neuroprotection in the specific context of neurodegeneration remains poorly explored. In this study behavioural, biochemical and cellular analyses were conducted to compare the effects of two different long-term exercise protocols, high intensity swimming and low intensity running, on motor units of a type 3 spinal muscular atrophy (SMA)-like mouse model. Our data revealed a preferential SMA-induced death of intermediate and fast motor neurons which was limited by the swimming protocol only, suggesting a close relationship between neuron-specific protection and their activation levels by specific exercise. The exercise-induced neuroprotection was independent of SMN protein expression and associated with specific metabolic and behavioural adaptations with notably a swimming-induced reduction of muscle fatigability. Our results provide new insight into the motor units' adaptations to different physical exercise parameters and will contribute to the design of new active physiotherapy protocols for patient care.
Abstract: Spinal muscular atrophy (SMA) is a group of autosomal recessive neurodegenerative diseases differing in their clinical outcome, characterized by the specific loss of spinal motor neurons, caused by insufficient level of expression of the protein survival of motor neuron (SMN). No cure is at present available for SMA. While physical exercise might represent a promising approach for alleviating SMA symptoms, the lack of data dealing with the effects of different exercise types on diseased motor units still precludes the use of active physiotherapy in SMA patients. In the present study, we have evaluated the efficiency of two long-term physical exercise paradigms, based on either high intensity swimming or low intensity running, in alleviating SMA symptoms in a mild type 3 SMA-like mouse model. We found that 10 months' physical training induced significant benefits in terms of resistance to muscle damage, energetic metabolism, muscle fatigue and motor behaviour. Both exercise types significantly enhanced motor neuron survival, independently of SMN expression, leading to the maintenance of neuromuscular junctions and skeletal muscle phenotypes, particularly in the soleus, plantaris and tibialis of trained mice. Most importantly, both exercises significantly improved neuromuscular excitability properties. Further, all these training-induced benefits were quantitatively and qualitatively related to the specific characteristics of each exercise, suggesting that the related neuroprotection is strongly dependent on the specific activation of some motor neuron subpopulations. Taken together, the present data show significant long-term exercise benefits in type 3 SMA-like mice providing important clues for designing rehabilitation programmes in patients.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


References
-
- Bacskai T, Rusznak Z, Paxinos G & Watson C (2014). Musculotopic organization of the motor neurons supplying the mouse hindlimb muscles: a quantitative study using Fluoro‐Gold retrograde tracing. Brain Struct Funct 219, 303–321. - PubMed
-
- Biondi O, Branchu J, Sanchez G, Lancelin C, Deforges S, Lopes P, Pariset C, Lecolle S, Cote J, Chanoine C & Charbonnier F (2010). In vivo NMDA receptor activation accelerates motor unit maturation, protects spinal motor neurons, and enhances SMN2 gene expression in severe spinal muscular atrophy mice. J Neurosci 30, 11288–11299. - PMC - PubMed
-
- Biondi O, Grondard C, Lecolle S, Deforges S, Pariset C, Lopes P, Cifuentes‐Diaz C, Li H, della Gaspera B, Chanoine C & Charbonnier F (2008). Exercise‐induced activation of NMDA receptor promotes motor unit development and survival in a type 2 spinal muscular atrophy model mouse. J Neurosci 28, 953–962. - PMC - PubMed
-
- Bladen CL, Thompson R, Jackson JM, Garland C, Wegel C, Ambrosini A, Pisano P, Walter MC, Schreiber O, Lusakowska A, Jedrzejowska M, Kostera‐Pruszczyk A, van der Pol L, Wadman RI, Gredal O, Karaduman A, Topaloglu H, Yilmaz O, Matyushenko V, Rasic VM, Kosac A, Karcagi V, Garami M, Herczegfalvi A, Monges S, Moresco A, Chertkoff L, Chamova T, Guergueltcheva V, Butoianu N, Craiu D, Korngut L, Campbell C, Haberlova J, Strenkova J, Alejandro M, Jimenez A, Ortiz GG, Enriquez GV, Rodrigues M, Roxburgh R, Dawkins H, Youngs L, Lahdetie J, Angelkova N, Saugier‐Veber P, Cuisset JM, Bloetzer C, Jeannet PY, Klein A, Nascimento A, Tizzano E, Salgado D, Mercuri E, Sejersen T, Kirschner J, Rafferty K, Straub V, Bushby K, Verschuuren J, Beroud C & Lochmuller H (2014). Mapping the differences in care for 5,000 spinal muscular atrophy patients, a survey of 24 national registries in North America, Australasia and Europe. J Neurol 261, 152–163. - PubMed
-
- Boerio D, Greensmith L & Bostock H (2009). Excitability properties of motor axons in the maturing mouse. J Peripher Nerv Syst 14, 45–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

