Vortex ring behavior provides the epigenetic blueprint for the human heart
- PMID: 26915473
- PMCID: PMC4768103
- DOI: 10.1038/srep22021
Vortex ring behavior provides the epigenetic blueprint for the human heart
Abstract
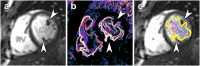
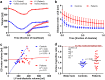
The laws of fluid dynamics govern vortex ring formation and precede cardiac development by billions of years, suggesting that diastolic vortex ring formation is instrumental in defining the shape of the heart. Using novel and validated magnetic resonance imaging measurements, we show that the healthy left ventricle moves in tandem with the expanding vortex ring, indicating that cardiac form and function is epigenetically optimized to accommodate vortex ring formation for volume pumping. Healthy hearts demonstrate a strong coupling between vortex and cardiac volumes (R(2) = 0.83), but this optimized phenotype is lost in heart failure, suggesting restoration of normal vortex ring dynamics as a new, and possibly important consideration for individualized heart failure treatment. Vortex ring volume was unrelated to early rapid filling (E-wave) velocity in patients and controls. Characteristics of vortex-wall interaction provide unique physiologic and mechanistic information about cardiac diastolic function that may be applied to guide the design and implantation of prosthetic valves, and have potential clinical utility as therapeutic targets for tailored medicine or measures of cardiac health.
Figures


References
-
- Liao J. C., Beal D. N., Lauder G. V. & Triantafyllou M. S. Fish exploiting vortices decrease muscle activity. Science 302, 1566–9 (2003). - PubMed
-
- Marten K., Shariff K., Psarakos S. & White D. J. Ring bubbles of dolphins. Sci. Am. 275, 82–7 (1996). - PubMed
-
- Hove J. R. et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421 (2003). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

