External Quality Assessment of MERS-CoV Molecular Diagnostics During the 2015 Korean Outbreak
- PMID: 26915611
- PMCID: PMC4773263
- DOI: 10.3343/alm.2016.36.3.230
External Quality Assessment of MERS-CoV Molecular Diagnostics During the 2015 Korean Outbreak
Abstract
Background: The largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection outside Middle East Asia in 2015 has necessitated the rapid expansion of laboratories that conduct MERS-CoV molecular testing in Korea, together with external quality assessment (EQA) to evaluate the assays used.
Methods: The EQA program consisted of two phases; self-validation and blind assessment. For the first EQA phase, in vitro transcribed upstream region of the envelope gene (upE) and the open reading frame (ORF)1a RNAs were used at a concentration of 1,000 copies/μL. The test panel for the second EQA phase consisted of RNA extracts from three samples, which were obtained from two MERS-CoV positive patients and one MERS-CoV negative patient.
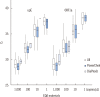
Results: The first EQA phase results for 46 participants showed a linear relationship between the threshold cycle (C(T)) values of RNA materials and the logarithmic concentrations for both upE and ORF1a gene targets (R²=0.73 and 0.75, respectively). The mean C(T) value for each concentration was different depending on which commercial kit was used for the assay. Among the three commonly used kits, PowerChek MERS Real-Time PCR kit (KogeneBiotech, Korea) showed the lowest C(T) values at all concentrations of upE and most concentrations of ORF1a. The second EQA phase results for 47 participants were 100% correct for all tested samples.
Conclusions: This EQA survey demonstrates that the MERS-CoV molecular testing performed in Korea during the 2015 outbreak is of robust capability. However, careful establishment and validation of a cut-off value are recommended to ensure good analytical sensitivity.
Keywords: External quality assessment; MERS-CoV; Molecular diagnostics; ORF1a; Real-time PCR; upE.
Conflict of interest statement
Figures
Similar articles
-
MERS-CoV diagnosis: An update.J Infect Public Health. 2016 May-Jun;9(3):216-9. doi: 10.1016/j.jiph.2016.04.005. Epub 2016 Apr 20. J Infect Public Health. 2016. PMID: 27106390 Free PMC article. Review.
-
Performance Evaluation of the PowerChek MERS (upE & ORF1a) Real-Time PCR Kit for the Detection of Middle East Respiratory Syndrome Coronavirus RNA.Ann Lab Med. 2017 Nov;37(6):494-498. doi: 10.3343/alm.2017.37.6.494. Ann Lab Med. 2017. PMID: 28840986 Free PMC article.
-
Analytical and Clinical Validation of Six Commercial Middle East Respiratory Syndrome Coronavirus RNA Detection Kits Based on Real-Time Reverse-Transcription PCR.Ann Lab Med. 2016 Sep;36(5):450-6. doi: 10.3343/alm.2016.36.5.450. Ann Lab Med. 2016. PMID: 27374710 Free PMC article.
-
First international external quality assessment of molecular diagnostics for Mers-CoV.J Clin Virol. 2015 Aug;69:81-5. doi: 10.1016/j.jcv.2015.05.022. Epub 2015 May 30. J Clin Virol. 2015. PMID: 26209385 Free PMC article.
-
Korean Society for Laboratory Medicine Practice Guidelines for the Molecular Diagnosis of Middle East Respiratory Syndrome During an Outbreak in Korea in 2015.Ann Lab Med. 2016 May;36(3):203-8. doi: 10.3343/alm.2016.36.3.203. Ann Lab Med. 2016. PMID: 26915607 Free PMC article. Review.
Cited by
-
MERS-CoV diagnosis: An update.J Infect Public Health. 2016 May-Jun;9(3):216-9. doi: 10.1016/j.jiph.2016.04.005. Epub 2016 Apr 20. J Infect Public Health. 2016. PMID: 27106390 Free PMC article. Review.
-
Proficiency testing for the detection of Middle East respiratory syndrome coronavirus demonstrates global capacity to detect Middle East respiratory syndrome coronavirus.J Med Virol. 2018 Dec;90(12):1827-1833. doi: 10.1002/jmv.25266. Epub 2018 Aug 27. J Med Virol. 2018. PMID: 30016543 Free PMC article.
-
Selection and Characterization of Monoclonal Antibodies Targeting Middle East Respiratory Syndrome Coronavirus through a Human Synthetic Fab Phage Display Library Panning.Antibodies (Basel). 2019 Jul 31;8(3):42. doi: 10.3390/antib8030042. Antibodies (Basel). 2019. PMID: 31544848 Free PMC article.
-
An updated roadmap for MERS-CoV research and product development: focus on diagnostics.BMJ Glob Health. 2019 Feb 1;4(Suppl 2):e001105. doi: 10.1136/bmjgh-2018-001105. eCollection 2019. BMJ Glob Health. 2019. PMID: 30815285 Free PMC article.
-
Performance Evaluation of the PowerChek MERS (upE & ORF1a) Real-Time PCR Kit for the Detection of Middle East Respiratory Syndrome Coronavirus RNA.Ann Lab Med. 2017 Nov;37(6):494-498. doi: 10.3343/alm.2017.37.6.494. Ann Lab Med. 2017. PMID: 28840986 Free PMC article.
References
-
- Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. - PubMed
-
- Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012;17:pii:20285. - PubMed
-
- Corman VM, Muller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012;17:pii:20334. - PubMed
-
- WHO. Laboratory Testing for Middle East respiratory syndrome coronavirus. Interim recommendations (revised) [Updated on Sep 2014]. http://www.who.int/csr/disease/coronavirus_infections/WHO_interim_recomm....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

