Cyclic nucleotide regulation of cardiac sympatho-vagal responsiveness
- PMID: 26915722
- PMCID: PMC4945711
- DOI: 10.1113/JP271827
Cyclic nucleotide regulation of cardiac sympatho-vagal responsiveness
Abstract
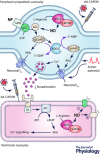
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are now recognized as important intracellular signalling molecules that modulate cardiac sympatho-vagal balance in the progression of heart disease. Recent studies have identified that a significant component of autonomic dysfunction associated with several cardiovascular pathologies resides at the end organ, and is coupled to impairment of cyclic nucleotide targeted pathways linked to abnormal intracellular calcium handling and cardiac neurotransmission. Emerging evidence also suggests that cyclic nucleotide coupled phosphodiesterases (PDEs) play a key role limiting the hydrolysis of cAMP and cGMP in disease, and as a consequence this influences the action of the nucleotide on its downstream biological target. In this review, we illustrate the action of nitric oxide-CAPON signalling and brain natriuretic peptide on cGMP and cAMP regulation of cardiac sympatho-vagal transmission in hypertension and ischaemic heart disease. Moreover, we address how PDE2A is now emerging as a major target that affects the efficacy of soluble/particulate guanylate cyclase coupling to cGMP in cardiac dysautonomia.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures




References
-
- Abramson BL, Ando S, Notarius CF, Rongen GA & Floras JS (1999). Effect of atrial natriuretic peptide on muscle sympathetic activity and its reflex control in human heart failure. Circulation 99, 1810–1815. - PubMed
-
- Ackermann U, Khanna J & Irizawa TG (1988). Atrial natriuretic factor alters autonomic interactions in the control of heart rate in conscious rats. Can J Physiol Pharmacol 66, 930–936. - PubMed
-
- Ashman DF, Lipton R, Melicow MM & Price TD (1963). Isolation of adenosine 3′,5′‐monophosphate and guanosine 3′,5′‐monophosphate from rat urine. Biochem Biophys Res Commun 11, 330–334. - PubMed
-
- Atchison DJ & Ackermann U (1993). The interaction between atrial natriuretic peptide and cardiac parasympathetic function. J Auton Nerv Syst 42, 81–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

