Hsp90 in Cancer: Transcriptional Roles in the Nucleus
- PMID: 26916002
- PMCID: PMC6790218
- DOI: 10.1016/bs.acr.2015.08.002
Hsp90 in Cancer: Transcriptional Roles in the Nucleus
Abstract
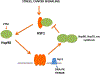
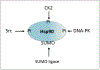
Hsp90 plays a key role in fostering metabolic pathways essential in tumorigenesis through its functions as a molecular chaperone. Multiple oncogenic factors in the membrane and cytoplasm are thus protected from degradation and destruction. Here, we have considered Hsp90's role in transcription in the nucleus. Hsp90 functions both in regulating the activity of sequence-specific transcription factors such as nuclear receptors and HSF1, as well as impacting more globally acting factors that act on chromatin and RNA polymerase II. Hsp90 influences transcription by modulating histone modification mediated by its clients SMYD3 and trithorax/MLL, as well as by regulating the processivity of RNA polymerase II through negative elongation factor. It is not currently clear how the transcriptional role of Hsp90 may be influenced by the cancer milieu although recently discovered posttranslational modification of the chaperone may be involved. Dysregulation of Hsp90 may thus influence malignant processes both by modulating the function of specific transcription factors and effects on more globally acting general components of the transcriptional machinery.
Keywords: Cancer transcription; Hsp90; Modification; Posttranslational; RNA polymerase II; Trithorax.
© 2016 Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Hsp90 as a "Chaperone" of the Epigenome: Insights and Opportunities for Cancer Therapy.Adv Cancer Res. 2016;129:107-40. doi: 10.1016/bs.acr.2015.09.003. Epub 2015 Nov 24. Adv Cancer Res. 2016. PMID: 26916003 Review.
-
Protein phosphatase 5 is a negative modulator of heat shock factor 1.J Biol Chem. 2005 Aug 12;280(32):28989-96. doi: 10.1074/jbc.M503594200. Epub 2005 Jun 20. J Biol Chem. 2005. PMID: 15967796
-
HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes.Mol Cell Biol. 1998 Sep;18(9):4949-60. doi: 10.1128/MCB.18.9.4949. Mol Cell Biol. 1998. PMID: 9710578 Free PMC article.
-
C-terminal domain of SMYD3 serves as a unique HSP90-regulated motif in oncogenesis.Oncotarget. 2015 Feb 28;6(6):4005-19. doi: 10.18632/oncotarget.2970. Oncotarget. 2015. PMID: 25738358 Free PMC article.
-
Posttranslational modification and beyond: interplay between histone deacetylase 6 and heat-shock protein 90.Mol Med. 2021 Sep 16;27(1):110. doi: 10.1186/s10020-021-00375-3. Mol Med. 2021. PMID: 34530730 Free PMC article. Review.
Cited by
-
Partners in Mischief: Functional Networks of Heat Shock Proteins of Plasmodium falciparum and Their Influence on Parasite Virulence.Biomolecules. 2019 Jul 23;9(7):295. doi: 10.3390/biom9070295. Biomolecules. 2019. PMID: 31340488 Free PMC article. Review.
-
Microarray-Based Screening of Putative HSP90 Inhibitors Predicted and Isolated from Microorganisms.Methods Mol Biol. 2022;2489:435-448. doi: 10.1007/978-1-0716-2273-5_22. Methods Mol Biol. 2022. PMID: 35524063
-
Methods to validate Hsp90 inhibitor specificity, to identify off-target effects, and to rethink approaches for further clinical development.Cell Stress Chaperones. 2018 Jul;23(4):467-482. doi: 10.1007/s12192-018-0877-2. Epub 2018 Feb 1. Cell Stress Chaperones. 2018. PMID: 29392504 Free PMC article. Review.
-
The functions and regulation of heat shock proteins; key orchestrators of proteostasis and the heat shock response.Arch Toxicol. 2021 Jun;95(6):1943-1970. doi: 10.1007/s00204-021-03070-8. Epub 2021 May 18. Arch Toxicol. 2021. PMID: 34003342 Review.
-
Disruption of DNA Repair and Survival Pathways through Heat Shock Protein Inhibition by Onalespib to Sensitize Malignant Gliomas to Chemoradiation Therapy.Clin Cancer Res. 2022 May 2;28(9):1979-1990. doi: 10.1158/1078-0432.CCR-20-0468. Clin Cancer Res. 2022. PMID: 35140124 Free PMC article.
References
-
- Bettermann K, Benesch M, Weis S, and Haybaeck J (2012). SUMOylation in carcinogenesis. Cancer Lett 316, 113–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

