Transmission of human mtDNA heteroplasmy in the Genome of the Netherlands families: support for a variable-size bottleneck
- PMID: 26916109
- PMCID: PMC4817766
- DOI: 10.1101/gr.203216.115
Transmission of human mtDNA heteroplasmy in the Genome of the Netherlands families: support for a variable-size bottleneck
Abstract
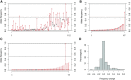
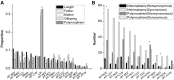
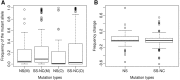
Although previous studies have documented a bottleneck in the transmission of mtDNA genomes from mothers to offspring, several aspects remain unclear, including the size and nature of the bottleneck. Here, we analyze the dynamics of mtDNA heteroplasmy transmission in the Genomes of the Netherlands (GoNL) data, which consists of complete mtDNA genome sequences from 228 trios, eight dizygotic (DZ) twin quartets, and 10 monozygotic (MZ) twin quartets. Using a minor allele frequency (MAF) threshold of 2%, we identified 189 heteroplasmies in the trio mothers, of which 59% were transmitted to offspring, and 159 heteroplasmies in the trio offspring, of which 70% were inherited from the mothers. MZ twin pairs exhibited greater similarity in MAF at heteroplasmic sites than DZ twin pairs, suggesting that the heteroplasmy MAF in the oocyte is the major determinant of the heteroplasmy MAF in the offspring. We used a likelihood method to estimate the effective number of mtDNA genomes transmitted to offspring under different bottleneck models; a variable bottleneck size model provided the best fit to the data, with an estimated mean of nine individual mtDNA genomes transmitted. We also found evidence for negative selection during transmission against novel heteroplasmies (in which the minor allele has never been observed in polymorphism data). These novel heteroplasmies are enhanced for tRNA and rRNA genes, and mutations associated with mtDNA diseases frequently occur in these genes. Our results thus suggest that the female germ line is able to recognize and select against deleterious heteroplasmies.
© 2016 Li et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
