Autophagy is activated to protect against endotoxic acute kidney injury
- PMID: 26916346
- PMCID: PMC4768171
- DOI: 10.1038/srep22171
Autophagy is activated to protect against endotoxic acute kidney injury
Abstract
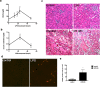
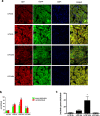
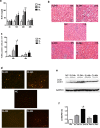
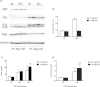
Endotoxemia in sepsis, characterized by systemic inflammation, is a major cause of acute kidney injury (AKI) in hospitalized patients, especially in intensive care unit; however the underlying pathogenesis is poorly understood. Autophagy is a conserved, cellular catabolic pathway that plays crucial roles in cellular homeostasis including the maintenance of cellular function and viability. The regulation and role of autophagy in septic or endotoxic AKI remains unclear. Here we show that autophagy was induced in kidney tubular cells in mice by the endotoxin lipopolysaccharide (LPS). Pharmacological inhibition of autophagy with chloroquine enhanced LPS-induced AKI. Moreover, specific ablation of autophagy gene 7 (Atg7) from kidney proximal tubules worsened LPS-induced AKI. Together, the results demonstrate convincing evidence of autophagy activation in endotoxic kidney injury and support a renoprotective role of autophagy in kidney tubules.
Figures

References
-
- Bone R. C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 101, 1644–1655 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

