Effects of biphasic, basal-bolus or basal insulin analogue treatments on carotid intima-media thickness in patients with type 2 diabetes mellitus: the randomised Copenhagen Insulin and Metformin Therapy (CIMT) trial
- PMID: 26916685
- PMCID: PMC4771974
- DOI: 10.1136/bmjopen-2015-008377
Effects of biphasic, basal-bolus or basal insulin analogue treatments on carotid intima-media thickness in patients with type 2 diabetes mellitus: the randomised Copenhagen Insulin and Metformin Therapy (CIMT) trial
Abstract
Objective: To assess the effect of 3 insulin analogue regimens on change in carotid intima-media thickness (IMT) in patients with type 2 diabetes.
Design and setting: Investigator-initiated, randomised, placebo-controlled trial with a 2 × 3 factorial design, conducted at 8 hospitals in Denmark.
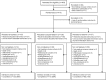
Participants and interventions: Participants with type 2 diabetes (glycated haemoglobin (HbA1c) ≥ 7.5% (≥ 58 mmol/mol), body mass index >25 kg/m(2)) were, in addition to metformin versus placebo, randomised to 18 months open-label biphasic insulin aspart 1-3 times daily (n=137) versus insulin aspart 3 times daily in combination with insulin detemir once daily (n=138) versus insulin detemir alone once daily (n=137), aiming at HbA1c ≤ 7.0% (≤ 53 mmol/mol).
Outcomes: Primary outcome was change in mean carotid IMT (a marker of subclinical cardiovascular disease). HbA1c, insulin dose, weight, and hypoglycaemic and serious adverse events were other prespecified outcomes.
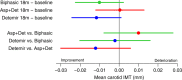
Results: Carotid IMT change did not differ between groups (biphasic -0.009 mm (95% CI -0.022 to 0.004), aspart+detemir 0.000 mm (95% CI -0.013 to 0.013), detemir -0.012 mm (95% CI -0.025 to 0.000)). HbA1c was more reduced with biphasic (-1.0% (95% CI -1.2 to -0.8)) compared with the aspart+detemir (-0.4% (95% CI -0.6 to -0.3)) and detemir (-0.3% (95% CI -0.4 to -0.1)) groups (p<0.001). Weight gain was higher in the biphasic (3.3 kg (95% CI 2.7 to 4.0) and aspart+detemir (3.2 kg (95% CI 2.6 to 3.9)) compared with the detemir group (1.9 kg (95% CI 1.3 to 2.6)). Insulin dose was higher with detemir (1.6 IU/kg/day (95% CI 1.4 to 1.8)) compared with biphasic (1.0 IU/kg/day (95% CI 0.9 to 1.1)) and aspart+detemir (1.1 IU/kg/day (95% CI 1.0 to 1.3)) (p<0.001). Number of participants with severe hypoglycaemia and serious adverse events did not differ.
Conclusions: Carotid IMT change did not differ between 3 insulin regimens despite differences in HbA1c, weight gain and insulin doses. The trial only reached 46% of planned sample size and lack of power may therefore have affected our results.
Trial registration number: NCT00657943.
Keywords: ULTRASONOGRAPHY.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/
Figures

References
-
- Inzucchi SE, Bergenstal RM, Buse JB et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. 10.1007/s00125-012-2534-0 - DOI - PubMed
-
- Abraira C, Colwell JA, Nuttall FQ et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care 1995;18:1113–23. 10.2337/diacare.18.8.1113 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
