Vancomycin Treatment Alters Humoral Immunity and Intestinal Microbiota in an Aged Mouse Model of Clostridium difficile Infection
- PMID: 26917573
- PMCID: PMC4907406
- DOI: 10.1093/infdis/jiw071
Vancomycin Treatment Alters Humoral Immunity and Intestinal Microbiota in an Aged Mouse Model of Clostridium difficile Infection
Abstract
Background: The elderly host is highly susceptible to severe disease and treatment failure in Clostridium difficile infection (CDI). We investigated how treatment with vancomycin in the aged host influences systemic and intestinal humoral responses and select intestinal microbiota.
Methods: Young (age, 2 months) and aged (age, 18 months) C57BL/6 mice were infected with VPI 10463 after exposure to broad-spectrum antibiotics. Vancomycin was given 24 hours after infection, and treatment was continued for 5 days. At select time points, specimens of serum and intestinal tissue and contents were collected for histopathologic analysis, to measure antibody levels and the pathogen burden, and to determine the presence and levels of select intestinal microbiota and C. difficile toxin.
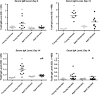
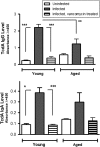
Results: Levels of disease severity, relapse, and mortality were increased, and recovery from infection was slower in aged mice compared to young mice. Serum levels of immunoglobulin M, immunoglobulin A, and immunoglobulin G against C. difficile toxin A were depressed in aged mice, and vancomycin treatment reduced antibody responses in both age groups. While baseline levels of total bacterial load, Bacteroidetes, Firmicutes, and Enterobacteriaceae were mostly similar, aged mice had a significant change in the Firmicutes to Bacteroidetes ratio with vancomycin treatment.
Conclusions: Vancomycin treatment decreases the systemic humoral response to CDI. Increased mortality from and recurrence of CDI in the aged host are associated with an impaired humoral response and a greater susceptibility to vancomycin-induced alteration of intestinal microbiota.
Keywords: Clostridium difficile infection; antibiotic-associated colitis; diarrhea; elderly.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures





References
-
- Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep 2011; 59:1–126. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

