A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine
- PMID: 26917574
- PMCID: PMC4907409
- DOI: 10.1093/infdis/jiw077
A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine
Abstract
Background: A novel translational pharmacology investigation was conducted by combining an in vitro efficacy target with mucosal tissue pharmacokinetic (PK) data and mathematical modeling to determine the number of doses required for effective human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP).
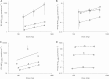
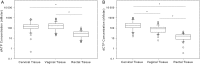
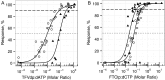
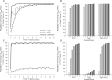
Methods: A PK/pharmacodynamic (PD) model was developed by measuring mucosal tissue concentrations of tenofovir, emtricitabine, their active metabolites (tenofovir diphosphate [TFVdp] and emtricitabine triphosphate [FTCtp], respectively), and competing endogenous nucleotides (dATP and dCTP) in 47 healthy women. TZM-bl and CD4(+) T cells were used to identify 90% effective concentration (EC90) ratios of TFVdp to dATP and FTCtp to dCTP (alone and in combination) for protection against HIV. Monte-Carlo simulations were then performed to identify minimally effective dosing strategies to protect lower female genital tract and colorectal tissues.
Results: The colorectal TFVdp concentration was 10 times higher than that in the lower female genital tract, whereas concentrations of endogenous nucleotides were 7-11 times lower. Our model predicted that ≥98% of the population achieved protective mucosal tissue exposure by the third daily dose of tenofovir disoproxil fumarate plus emtricitabine. However, a minimum adherence to 6 of 7 doses/week (85%) was required to protect lower female genital tract tissue from HIV, while adherence to 2 of 7 doses/week (28%) was required to protect colorectal tissue.
Conclusions: This model is predictive of recent PrEP trial results in which 2-3 doses/week was 75%-90% effective in men but ineffective in women. These data provide a novel approach for future PrEP investigations that can optimize clinical trial dosing strategies.
Keywords: HIV; antiretroviral; dose response; population pharmacokinetics-pharmacodynamics; preexposure prophylaxis; quantitative pharmacology; translational medicine.
© The Author 2016. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail journals.permissions@oup.com.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

