Serum periostin in obstructive airways disease
- PMID: 26917610
- PMCID: PMC4851937
- DOI: 10.1183/13993003.01384-2015
Serum periostin in obstructive airways disease
Abstract
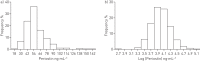
Serum periostin is a potential biomarker of response to therapies that target type 2 inflammation in asthma. The objectives of this study were to describe: 1) the distribution of serum periostin levels in adults with symptomatic airflow obstruction; 2) its relationship with other variables, including type 2 biomarkers; and 3) the effect of inhaled corticosteroids on periostin levels.Serum periostin levels were measured in a cross-sectional study exploring phenotypes and biomarkers in 386 patients aged 18-75 years who reported wheeze and breathlessness in the past 12 months. In 49 ICS-naïve patients, periostin levels were measured again after 12 weeks of budesonide (800 μg·day(-1)).The distribution of serum periostin levels was right skewed (mean±sd 57.3±18.6 ng·mL(-1), median (interquartile range) 54.0 (45.1-65.6) ng·mL(-1), range 15.0-164.7 ng·mL(-1)). Periostin was positively associated with exhaled nitric oxide (Spearman's rho=0.22, p<0.001), blood eosinophil count (Spearman's rho=0.21, p<0.001), and total IgE (Spearman's rho=0.14, p=0.007). The Hodges-Lehmann estimator (95% CI) of change in periostin level after ICS therapy was -4.8 (-6.7- -3.2) ng·mL(-1) (p<0.001).These findings provide data on the distribution of serum periostin in adults with symptomatic airflow obstruction, the weak associations between periostin and other type 2 markers, and the reduction in periostin with inhaled corticosteroid therapy.
Copyright ©ERS 2016.
Figures

References
-
- Taylor DR. Using biomarkers in the assessment of airways disease. J Allergy Clin Immunol 2011; 128: 927–934. - PubMed
-
- Petsky HL, Cates CJ, Lasserson TJ, et al. . A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax 2012; 67: 199–208. - PubMed
-
- Malerba M, Ragnoli B, Radaeli A, et al. . Usefulness of exhaled nitric oxide and sputum eosinophils in the long-term control of eosinophilic asthma. Chest 2008; 134: 733–739. - PubMed
-
- Castro M, Mathur S, Hargreave F, et al. . Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125–1132. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
