Evaluation of the Tolerability of Switching Patients on Chronic Full μ-Opioid Agonist Therapy to Buccal Buprenorphine
- PMID: 26917621
- PMCID: PMC4984426
- DOI: 10.1093/pm/pnv110
Evaluation of the Tolerability of Switching Patients on Chronic Full μ-Opioid Agonist Therapy to Buccal Buprenorphine
Abstract
Objective: Assess whether patients with chronic pain receiving 80 to 220 mg oral morphine sulfate equivalent of a full Μ: -opioid agonist could be transitioned to buccal buprenorphine at approximately 50% of their full dose without inducing opioid withdrawal or sacrificing analgesic efficacy.
Methods: A randomized, double-blind, double-dummy, active-controlled, two-period crossover study in adult patients receiving around-the-clock full opioid agonist therapy and confirmed to be opioid dependent by naloxone challenge. Study doses were substituted at the time of the regular dose schedule for each patient. The primary endpoint was the proportion of patients with a maximum Clinical Opiate Withdrawal Scale score ≥ 13 (moderate withdrawal) or use of rescue medication.
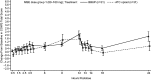
Results: 35 subjects on ≥ 80 mg morphine sulfate equivalent per day were evaluable for opioid withdrawal. One patient during buccal buprenorphine treatment and two during 50% full Μ: -opioid agonist treatment experienced opioid withdrawal of at least moderate intensity. The mean maximum Clinical Opiate Withdrawal Scale scores were similar, and numerically lower on buccal buprenorphine. There were no significant differences in pain ratings between treatments. The most frequent adverse events with buccal buprenorphine were headache (19%), vomiting (13%), nausea, diarrhea, and drug withdrawal syndrome (each 9%), and with full Μ: -opioid agonist were headache (16%), drug withdrawal syndrome (13%), and nausea (6%).
Conclusions: Chronic pain patients treated with around-the-clock full Μ: -opioid agonist therapy can be switched to buccal buprenorphine (a partial Μ: -opioid agonist) at approximately 50% of the full Μ: -opioid agonist dose without an increased risk of opioid withdrawal or loss of pain control.
Keywords: Buprenorphine Buccal Film; Chronic Pain; Opioid.
© 2016 American Academy of Pain Medicine.
Figures



References
-
- Kajiwara M, Aoki K, Ishii K, et al. Agonist and antagonist actions of buprenorphine on three types of opioid receptor in isolated preparations. Jpn J Pharmacol 1986;40:95–101. - PubMed
-
- Leander JD. Buprenorphine is a potent kappa-opioid receptor antagonist in pigeons and mice. Eur J Pharmacol 1988;151:457–61. - PubMed
-
- Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine: Norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther 2001;297:688–95. - PubMed
-
- Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther 1995;274:361–72. - PubMed
-
- Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol 2012;10:209–19. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

