Presentation of Benefits and Harms in US Cancer Screening and Prevention Guidelines: Systematic Review
- PMID: 26917630
- PMCID: PMC5009951
- DOI: 10.1093/jnci/djv436
Presentation of Benefits and Harms in US Cancer Screening and Prevention Guidelines: Systematic Review
Abstract
Background: Cancer prevention and screening guidelines are ideally suited to the task of providing high-quality benefit-harm information that informs clinical practice. We systematically examined how US guidelines present benefits and harms for recommended cancer prevention and screening interventions.
Methods: We included cancer screening and prevention recommendations from: 1) the United States Preventive Services Task Force, 2) the American Cancer Society, 3) the American College of Physicians, 4) the National Comprehensive Cancer Network, and 5) other US guidelines within the National Guidelines Clearinghouse. Searches took place November 20, 2013, and January 1, 2014, and updates were reviewed through July 1, 2015. Two coders used an abstraction form to code information about benefits and harms presented anywhere within a guideline document, including appendices. The primary outcome was each recommendation's benefit-harm "comparability" rating, based on how benefits and harms were presented. Recommendations presenting absolute effects for both benefits and harms received a "comparable" rating. Other recommendations received an incomplete rating or an asymmetric rating based on prespecified criteria.
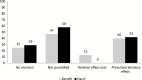
Results: Fifty-five recommendations for using interventions to prevent or detect breast, prostate, colon, cervical, and lung cancer were identified among 32 guidelines. Thirty point nine percent (n = 17) received a comparable rating, 14.5% (n = 8) received an incomplete rating, and 54.5% (n = 30) received an asymmetric rating.
Conclusions: Sixty-nine percent of cancer prevention and screening recommendation statements either did not quantify benefits and harms or presented them in an asymmetric manner. Improved presentation of benefits and harms in guidelines would better ensure that clinicians and patients have access to the information required for making informed decisions.
Published by Oxford University Press 2016. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

Comment in
-
Presenting Benefits and Downsides to Facilitate High-Quality Decision-Making About Cancer Screening.J Natl Cancer Inst. 2016 Feb 24;108(6):djv433. doi: 10.1093/jnci/djv433. Print 2016 Jun. J Natl Cancer Inst. 2016. PMID: 26917629 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

