Composition, Assembly, and Trafficking of a Wheat Xylan Synthase Complex
- PMID: 26917684
- PMCID: PMC4825154
- DOI: 10.1104/pp.15.01777
Composition, Assembly, and Trafficking of a Wheat Xylan Synthase Complex
Abstract
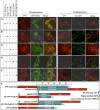
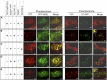
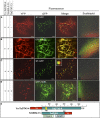
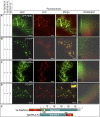
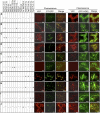
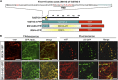
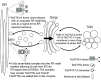
Xylans play an important role in plant cell wall integrity and have many industrial applications. Characterization of xylan synthase (XS) complexes responsible for the synthesis of these polymers is currently lacking. We recently purified XS activity from etiolated wheat (Triticum aestivum) seedlings. To further characterize this purified activity, we analyzed its protein composition and assembly. Proteomic analysis identified six main proteins: two glycosyltransferases (GTs) TaGT43-4 and TaGT47-13; two putative mutases (TaGT75-3 and TaGT75-4) and two non-GTs; a germin-like protein (TaGLP); and a vernalization related protein (TaVER2). Coexpression of TaGT43-4, TaGT47-13, TaGT75-3, and TaGT75-4 in Pichia pastoris confirmed that these proteins form a complex. Confocal microscopy showed that all these proteins interact in the endoplasmic reticulum (ER) but the complexes accumulate in Golgi, and TaGT43-4 acts as a scaffold protein that holds the other proteins. Furthermore, ER export of the complexes is dependent of the interaction between TaGT43-4 and TaGT47-13. Immunogold electron microscopy data support the conclusion that complex assembly occurs at specific areas of the ER before export to the Golgi. A di-Arg motif and a long sequence motif within the transmembrane domains were found conserved at the NH2-terminal ends of TaGT43-4 and homologous proteins from diverse taxa. These conserved motifs may control the forward trafficking of the complexes and their accumulation in the Golgi. Our findings indicate that xylan synthesis in grasses may involve a new regulatory mechanism linking complex assembly with forward trafficking and provide new insights that advance our understanding of xylan biosynthesis and regulation in plants.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures






References
-
- Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA III, Orlando R, Scheller HV, Mohnen D (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc Natl Acad Sci USA 108: 20225–20230 - PMC - PubMed
-
- Barlowe C. (2003) Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol 13: 295–300 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

