Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein
- PMID: 26917774
- PMCID: PMC4898656
- DOI: 10.1126/science.aad4234
Direct CRISPR spacer acquisition from RNA by a natural reverse transcriptase-Cas1 fusion protein
Abstract
CRISPR systems mediate adaptive immunity in diverse prokaryotes. CRISPR-associated Cas1 and Cas2 proteins have been shown to enable adaptation to new threats in type I and II CRISPR systems by the acquisition of short segments of DNA (spacers) from invasive elements. In several type III CRISPR systems, Cas1 is naturally fused to a reverse transcriptase (RT). In the marine bacterium Marinomonas mediterranea (MMB-1), we showed that a RT-Cas1 fusion protein enables the acquisition of RNA spacers in vivo in a RT-dependent manner. In vitro, the MMB-1 RT-Cas1 and Cas2 proteins catalyze the ligation of RNA segments into the CRISPR array, which is followed by reverse transcription. These observations outline a host-mediated mechanism for reverse information flow from RNA to DNA.
Copyright © 2016, American Association for the Advancement of Science.
Figures



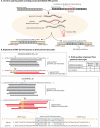
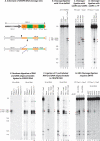

Comment in
-
RNA. CRISPR goes retro.Science. 2016 Feb 26;351(6276):920-1. doi: 10.1126/science.aaf2851. Science. 2016. PMID: 26917756 No abstract available.
-
Microbial genetics: CRISPR memories of RNA.Nat Rev Genet. 2016 Apr;17(4):192-3. doi: 10.1038/nrg.2016.31. Epub 2016 Mar 7. Nat Rev Genet. 2016. PMID: 26948816 No abstract available.
References
-
- Barrangou R, et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science. 2007;315:1709–1712. - PubMed
-
- Bolotin A, Quinquis B, Sorokin A, Ehrlich SD. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005;151:2551–2561. - PubMed
-
- Mojica FJ, Diez-Villasenor C, Garcia-Martinez J, Soria E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J Mol Evol. 2005;60:174–182. - PubMed
-
- Pourcel C, Salvignol G, Vergnaud G. CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies. Microbiology. 2005;151:653–663. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

