Capturing relevant extracellular matrices for investigating cell migration
- PMID: 26918156
- PMCID: PMC4754037
- DOI: 10.12688/f1000research.6623.1
Capturing relevant extracellular matrices for investigating cell migration
Abstract
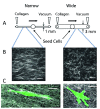
Much progress in understanding cell migration has been determined by using classic two-dimensional (2D) tissue culture platforms. However, increasingly, it is appreciated that certain properties of cell migration in vivo are not represented by strictly 2D assays. There is much interest in creating relevant three-dimensional (3D) culture environments and engineered platforms to better represent features of the extracellular matrix and stromal microenvironment that are not captured in 2D platforms. Important to this goal is a solid understanding of the features of the extracellular matrix-composition, stiffness, topography, and alignment-in different tissues and disease states and the development of means to capture these features.
Keywords: ECM; Matrix remodeling; cell migration; extracellular matrix; stromal microenvironment.
Conflict of interest statement
No competing interests were disclosed.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

