Human IgG1 antibodies suppress angiogenesis in a target-independent manner
- PMID: 26918197
- PMCID: PMC4763941
- DOI: 10.1038/sigtrans.2015.1
Human IgG1 antibodies suppress angiogenesis in a target-independent manner
Abstract
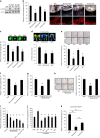
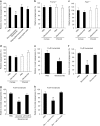
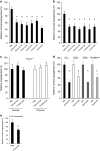
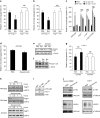
Aberrant angiogenesis is implicated in diseases affecting nearly 10% of the world's population. The most widely used anti-angiogenic drug is bevacizumab, a humanized IgG1 monoclonal antibody that targets human VEGFA. Although bevacizumab does not recognize mouse Vegfa, it inhibits angiogenesis in mice. Here we show bevacizumab suppressed angiogenesis in three mouse models not via Vegfa blockade but rather Fc-mediated signaling through FcγRI (CD64) and c-Cbl, impairing macrophage migration. Other approved humanized or human IgG1 antibodies without mouse targets (adalimumab, alemtuzumab, ofatumumab, omalizumab, palivizumab and tocilizumab), mouse IgG2a, and overexpression of human IgG1-Fc or mouse IgG2a-Fc, also inhibited angiogenesis in wild-type and FcγR humanized mice. This anti-angiogenic effect was abolished by Fcgr1 ablation or knockdown, Fc cleavage, IgG-Fc inhibition, disruption of Fc-FcγR interaction, or elimination of FcRγ-initated signaling. Furthermore, bevacizumab's Fc region potentiated its anti-angiogenic activity in humanized VEGFA mice. Finally, mice deficient in FcγRI exhibited increased developmental and pathological angiogenesis. These findings reveal an unexpected anti-angiogenic function for FcγRI and a potentially concerning off-target effect of hIgG1 therapies.
Conflict of interest statement
JA is a co-founder of iVeena Holdings, iVeena Pharmaceuticals, iVeena Delivery Systems and Inflammasome Therapeutics, and has received honoraria from Allergan and research funding from Olix Pharmaceuticals unrelated to this work. JA and SDF are named as inventors on patent applications filed by the University of Kentucky relating to the technology described in this work. MRC is listed as an inventor on patents covering alemtuzumab and MRC and KLA are listed as inventors on patents covering the Fc mutated forms of alemtuzumab.
Figures


References
-
- Nelson AL , Dhimolea E , Reichert JM . Development trends for human monoclonal antibody therapeutics. Nat Rev Drug Discov 2010; 9: 767–774. - PubMed
-
- Presta LG , Chen H , O'Connor SJ , Chisholm V , Meng YG , Krummen L et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res 1997; 57: 4593–4599. - PubMed
-
- Ferrara N , Hillan KJ , Gerber HP , Novotny W . Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004; 3: 391–400. - PubMed
-
- Liang WC , Wu X , Peale FV , Lee CV , Meng YG , Gutierrez J et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 2006; 281: 951–961. - PubMed
Publication types
Grants and funding
- R01 EY017182/EY/NEI NIH HHS/United States
- RC1 EY020442/EY/NEI NIH HHS/United States
- R00 EY024336/EY/NEI NIH HHS/United States
- R01 EY018350/EY/NEI NIH HHS/United States
- K99 EY024336/EY/NEI NIH HHS/United States
- UL1 RR033173/RR/NCRR NIH HHS/United States
- R21 EY019778/EY/NEI NIH HHS/United States
- R01 EY018836/EY/NEI NIH HHS/United States
- R01 EY020672/EY/NEI NIH HHS/United States
- T32 HL091812/HL/NHLBI NIH HHS/United States
- R01 EY024068/EY/NEI NIH HHS/United States
- R01 EY017950/EY/NEI NIH HHS/United States
- DP1 GM114862/GM/NIGMS NIH HHS/United States
- R01 EY022238/EY/NEI NIH HHS/United States
- K08 EY021521/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

