Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor
- PMID: 26918260
- PMCID: PMC4966836
- DOI: 10.1080/19420862.2015.1136043
Preclinical pharmacokinetics, pharmacodynamics, tissue distribution, and tumor penetration of anti-PD-L1 monoclonal antibody, an immune checkpoint inhibitor
Abstract
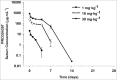
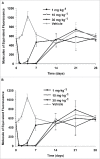
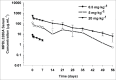
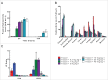
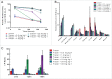
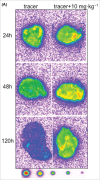
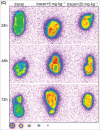
MPDL3280A is a human monoclonal antibody that targets programmed cell death-1 ligand 1 (PD-L1), and exerts anti-tumor activity mainly by blocking PD-L1 interaction with programmed cell death-1 (PD-1) and B7.1. It is being investigated as a potential therapy for locally advanced or metastatic malignancies. The purpose of the study reported here was to characterize the pharmacokinetics, pharmacodynamics, tissue distribution and tumor penetration of MPDL3280A and/or a chimeric anti-PD-L1 antibody PRO304397 to help further clinical development. The pharmacokinetics of MPDL3280A in monkeys at 0.5, 5 and 20 mg · kg(-1) and the pharmacokinetics / pharmacodynamics of PRO304397 in mice at 1, 3 10 mg · kg(-1) were determined after a single intravenous dose. Tissue distribution and tumor penetration for radiolabeled PRO304397 in tumor-bearing mouse models were determined. The pharmacokinetics of MPDL3280A and PRO304397 were nonlinear in monkeys and mice, respectively. Complete saturation of PD-L1 in blood in mice was achieved at serum concentrations of PRO304397 above ∼ 0.5 µg · mL(-1). Tissue distribution and tumor penetration studies of PRO304397 in tumor-bearing mice indicated that the minimum tumor interstitial to plasma radioactivity ratio was ∼ 0.3; saturation of target-mediated uptake in non-tumor tissues and desirable exposure in tumors were achieved at higher serum concentrations, and the distribution into tumors was dose-and time-dependent. The biodistribution data indicated that the efficacious dose is mostly likely higher than that estimated based on simple pharmacokinetics/pharmacodynamics in blood. These data also allowed for estimation of the target clinical dose for further development of MPDL3280A.
Keywords: Anti-PD-L1; PD-L1; pharmacodynamics; pharmacokinetics; tissue distribution; tumor penetration.
Figures

References
-
- Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, et al.. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381-5; PMID:16585157; http://dx.doi.org/10.1158/0008-5472.CAN-05-4303 - DOI - PubMed
-
- Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/10.1002/cncr.24899 - DOI - PubMed
-
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, et al.. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007; 104:3360-5; PMID:17360651; http://dx.doi.org/10.1073/pnas.0611533104 - DOI - PMC - PubMed
-
- Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother 2005; 54:307-14; PMID:15599732; http://dx.doi.org/10.1007/s00262-004-0593-x - DOI - PMC - PubMed
-
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008; 26:677-704; PMID:18173375; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090331 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
