Effect of Saccharomyces boulardii and Mode of Delivery on the Early Development of the Gut Microbial Community in Preterm Infants
- PMID: 26918330
- PMCID: PMC4769247
- DOI: 10.1371/journal.pone.0150306
Effect of Saccharomyces boulardii and Mode of Delivery on the Early Development of the Gut Microbial Community in Preterm Infants
Abstract
Background: Recent advances in culture-independent approaches have enabled insights into the diversity, complexity, and individual variability of gut microbial communities.
Objectives: To examine the effect of oral administration of Saccharomyces (S.) boulardii and mode of delivery on the intestinal microbial community in preterm infants.
Study design: Stool samples were collected from preterm newborns randomly divided into two groups: a probiotic-receiving group (n = 18) or a placebo group (n = 21). Samples were collected before probiotic intake (day 0), and after 2 and 6 weeks of supplementation. The composition of colonizing bacteria was assessed by 16S ribosomal RNA (rRNA) gene sequencing of fecal samples using the Ion 16S Metagenomics Kit and the Ion Torrent Personal Genome Machine platform.
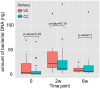
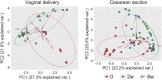
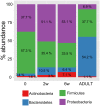
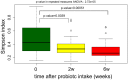
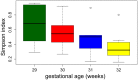
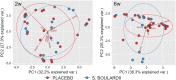
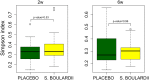
Results: A total of 11932257 reads were generated, and were clustered into 459, 187, and 176 operational taxonomic units at 0 days, 2 weeks, and 6 weeks, respectively. Of the 17 identified phyla, Firmicutes Actinobacteria, Proteobacteria, and Bacteroidetes were universal. The microbial community differed at day 0 compared with at 2 weeks and 6 weeks. There was a tendency for increased bacterial diversity at 2 weeks and 6 weeks compared with day 0, and infants with a gestational age of 31 weeks or higher presented increased bacterial diversity prior to S. boulardii administration. Firmicutes and Proteobacteria remained stable during the observation period, whereas Actinobacteria and Bacteroidetes increased in abundance, the latter particularly more sharply in vaginally delivered infants.
Conclusion: While the mode of delivery may influence the development of a microbial community, this study had not enough power to detect statistical differences between cohorts supplemented with probiotics, and in a consequence, to speculate on S. boulardii effect on gut microbiome composition in preterm newborns.
Conflict of interest statement
Figures
References
-
- Blaut M, Clavel T. Metabolic diversity of the intestinal microbiota: implications for health and disease. J Nutr. 2007;137: 751S–5S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

