The use of lectin microarray for assessing glycosylation of therapeutic proteins
- PMID: 26918373
- PMCID: PMC4966825
- DOI: 10.1080/19420862.2016.1149662
The use of lectin microarray for assessing glycosylation of therapeutic proteins
Abstract
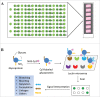
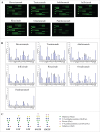
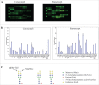
Glycans or carbohydrates attached to therapeutic glycoproteins can directly affect product quality, safety and efficacy, and therefore must be adequately analyzed and controlled throughout product life cycles. However, the complexity of protein glycosylation poses a daunting analytical challenge. In this study, we evaluated the utility of a lectin microarray for assessing protein glycans. Using commercial lectin chips, which contain 45 lectins toward distinct glycan structures, we were able to determine the lectin binding patterns of a panel of 15 therapeutic proteins, including 8 monoclonal antibodies. Lectin binding signals were analyzed to generate glycan profiles that were generally consistent with the known glycan patterns for these glycoproteins. In particular, the lectin-based microarray was found to be highly sensitive to variations in the terminal carbohydrate structures such as galactose versus sialic acid epitopes. These data suggest that lectin microarray could be used for screening glycan patterns of therapeutic glycoproteins.
Keywords: Glycan analysis; lectin microarray; monoclonal antibodies; therapeutic glycoproteins.
Figures



References
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem 1985; 54:631-64; PMID:3896128; http://dx.doi.org/10.1146/annurev.bi.54.070185.003215 - DOI - PubMed
-
- Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev 2012; 28:147-75; PMID:22616486; http://dx.doi.org/10.5661/bger-28-147 - DOI - PubMed
-
- Sola RJ, Griebenow K. Glycosylation of therapeutic proteins: an effective strategy to optimize efficacy. BioDrugs 2010; 24:9-21; PMID:20055529; http://dx.doi.org/10.2165/11530550-000000000-00000 - DOI - PMC - PubMed
-
- Sola RJ, Griebenow K. Effects of glycosylation on the stability of protein pharmaceuticals. J Pharm Sci 2009; 98:1223-45; PMID:18661536; http://dx.doi.org/10.1002/jps.21504 - DOI - PMC - PubMed
-
- Narhi LO, Arakawa T, Aoki KH, Elmore R, Rohde MF, Boone T, Strickland TW. The effect of carbohydrate on the structure and stability of erythropoietin. J Biol Chem 1991; 266:23022-6; PMID:1744097 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
