Unfolding of a Temperature-Sensitive Domain Controls Voltage-Gated Channel Activation
- PMID: 26919429
- PMCID: PMC4769381
- DOI: 10.1016/j.cell.2016.02.001
Unfolding of a Temperature-Sensitive Domain Controls Voltage-Gated Channel Activation
Abstract
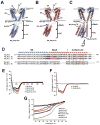
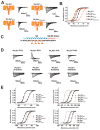
Voltage-gated ion channels (VGICs) are outfitted with diverse cytoplasmic domains that impact function. To examine how such elements may affect VGIC behavior, we addressed how the bacterial voltage-gated sodium channel (BacNa(V)) C-terminal cytoplasmic domain (CTD) affects function. Our studies show that the BacNa(V) CTD exerts a profound influence on gating through a temperature-dependent unfolding transition in a discrete cytoplasmic domain, the neck domain, proximal to the pore. Structural and functional studies establish that the BacNa(V) CTD comprises a bi-partite four-helix bundle that bears an unusual hydrophilic core whose integrity is central to the unfolding mechanism and that couples directly to the channel activation gate. Together, our findings define a general principle for how the widespread four-helix bundle cytoplasmic domain architecture can control VGIC responses, uncover a mechanism underlying the diverse BacNa(V) voltage dependencies, and demonstrate that a discrete domain can encode the temperature-dependent response of a channel.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
-
- Berova N, Nakanishi K, Woody RW. Circular Dichroism: Principles and Applications. 2. New York: Wiley-VCH; 2000.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

