Soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1) Is Elevated in Bronchoalveolar Lavage Fluid of Patients with Acute Respiratory Distress Syndrome
- PMID: 26919714
- PMCID: PMC4768838
- DOI: 10.1371/journal.pone.0149687
Soluble Vascular Cell Adhesion Molecule-1 (sVCAM-1) Is Elevated in Bronchoalveolar Lavage Fluid of Patients with Acute Respiratory Distress Syndrome
Abstract
Introduction: Pulmonary vascular endothelial activation has been implicated in acute respiratory distress syndrome (ARDS), yet little is known about the presence and role of endothelial activation markers in the alveolar space in ARDS. We hypothesized that endothelial activation biomarkers would be differentially expressed in bronchoalveolar lavage fluid from patients with ARDS compared with healthy volunteers, and that biomarker concentrations would be associated with ARDS severity.
Methods: We performed a cross-sectional analysis of data from 26 intubated patients with ARDS undergoing evaluation for clinically suspected ventilator-associated pneumonia and five healthy volunteers. Patients underwent bronchoalveolar lavage a median of five days after intubation. Healthy volunteers also underwent bronchoalveolar lavage. Endothelial activation biomarkers (soluble vascular cell adhesion molecule-1 [sVCAM-1], soluble endothelial selectin [sESEL], angiopoietin-1 [Ang-1] and angiopoietin-2 [Ang-2]) were measured in bronchoalveolar lavage fluid. Clinically suspected ventilator-associated pneumonia was confirmed with microbiologic culture data.
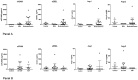
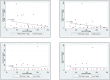
Results: Patients with ARDS had significantly higher median sVCAM-1 concentrations in the bronchoalveolar lavage fluid compared with healthy volunteers (985 vs 119 pg/mL, p = 0.03). Additionally, there was a trend toward greater bronchoalveolar lavage fluid sVCAM-1 concentrations among patients with moderate/severe compared to mild ARDS (1395 vs 209 pg/mL, p = 0.06). We did not detect significant differences in bronchoalveolar lavage fluid levels of sESEL, Ang-1 or Ang-2 between patients with ARDS and healthy volunteers. Median bronchoalveolar lavage fluid biomarker levels did not differ between patients with and without microbiologically-confirmed ventilator-associated pneumonia.
Conclusions: sVCAM-1 concentrations were significantly higher in the bronchoalveolar lavage fluid of patients with ARDS compared to healthy controls, and tended to be higher in moderate/severe ARDS compared to mild ARDS. Our findings add to the growing evidence supporting the concept that endothelial activation plays an important mechanistic role in the pathogenesis of ARDS. Further studies are necessary to characterize the role and/or clinical significance of sVCAM-1 and other endothelial activation markers present in the alveolar space in ARDS.
Conflict of interest statement
Figures
References
-
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005; 353:1685–1693. - PubMed
-
- Orfanos SE, Mavrommati I, Korovesi I, Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med 2004; 30:1702–1714. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

