Envelope Structures of Gram-Positive Bacteria
- PMID: 26919863
- PMCID: PMC5002265
- DOI: 10.1007/82_2015_5021
Envelope Structures of Gram-Positive Bacteria
Abstract
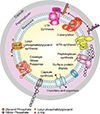
Gram-positive organisms, including the pathogens Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis, have dynamic cell envelopes that mediate interactions with the environment and serve as the first line of defense against toxic molecules. Major components of the cell envelope include peptidoglycan (PG), which is a well-established target for antibiotics, teichoic acids (TAs), capsular polysaccharides (CPS), surface proteins, and phospholipids. These components can undergo modification to promote pathogenesis, decrease susceptibility to antibiotics and host immune defenses, and enhance survival in hostile environments. This chapter will cover the structure, biosynthesis, and important functions of major cell envelope components in gram-positive bacteria. Possible targets for new antimicrobials will be noted.
Figures




References
-
- Abachin E, Poyart C, Pellegrini E, Milohanic E, Fiedler F, Berche P, Trieu-Cuot P. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulene of Listeria monocytogenes. Mol Microbiol. 2002;43:1–14. - PubMed
-
- Abee T, Kovács AT, Kuipers, van der Veen A. Biofilm formation and dispersal in Gram-positive bacteria. Curr Opin Biotechnol. 2011;22:172–179. - PubMed
-
- Allison SE, D’Elia MA, Arar S, Monteiro MA, Brown ED. Studies of the genetics, function, and kinetic mechanism of TagE, the wall teichoic acid glycosyltransferase in Bacillus subtilis 168. J Biol Chem. 2011;286(27):23708–23716. http://doi.org/10.1074/jbc.M111.241265. - DOI - PMC - PubMed
-
- Aly R, Shinefield HR, Litz C, Maibach HI. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J Infect Dis. 1980;141(4):463–465. - PubMed
-
- Anderson JS, Matsuhashi M, Haskin MA, Strominger JL. Biosynthesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967;242(13):3180–3190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

