A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells
- PMID: 26920032
- PMCID: PMC4899127
- DOI: 10.1007/s13402-016-0272-x
A novel curcumin-like dienone induces apoptosis in triple-negative breast cancer cells
Abstract
Purpose: According to the World Health Organization (WHO), breast cancer is the most common cancer affecting women worldwide. In the USA ~12.3 % of all women are expected to be diagnosed with various types of breast cancer, exhibiting varying degrees of therapeutic response rates. Therefore, the identification of novel anti-breast cancer drugs is of paramount importance.
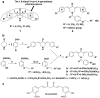
Methods: The 1,5-diaryl-3-oxo-1,4-pentadienyl pharmacophore was incorporated into a number of cytotoxins. Three of the resulting dienones, 2a, 2b and 2c, were tested for their anti-neoplastic potencies in a variety of human breast cancer-derived cell lines, including the triple negative MDA-MB-231 cell line and its metastatic variant, using a live-cell bio-imaging method. Special emphasis was put on dienone 2c, since its anti-cancer activity and its mode of inflicting cell death have so far not been reported.
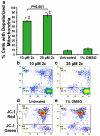
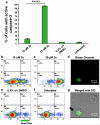
Results: We found that all three dienones exhibited potent cytotoxicities towards the breast cancer-derived cell lines tested, whereas significantly lower toxicities were observed towards the non-cancerous human breast cell line MCF-10A. The dienones 2b and 2c exhibited the greatest selective cytotoxicity at submicromolar concentration levels. We found that these two dienones induced phosphatidylserine externalization in MDA-MB-231 cells in a concentration-dependent manner, suggesting that their cytotoxic effect might be mediated by apoptosis. This possibility was confirmed by our observation that the dienone 2c can induce mitochondrial depolarization, caspase-3 activation, cell cycle disruption and DNA fragmentation in MDA-MB-231 cells.
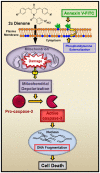
Conclusion: Our findings indicate that dienone 2c uses the mitochondrial/intrinsic pathway to inflict apoptosis in triple negative MDA-MB-231 breast cancer-derived cells. This observation warrants further assessment of dienone 2c as a potential anti-breast cancer drug.
Keywords: Anti-cancer drug discovery; Apoptosis; Caspase-3; Cell cycle; Curcumin analogues; DNA fragmentation; Mitochondrial depolarization.
Figures



Similar articles
-
Tumor-selective cytotoxicity of a novel pentadiene analogue on human leukemia/ lymphoma cells.Clin Cancer Drugs. 2016;3(2):138-146. doi: 10.2174/2212697X03666160830165250. Clin Cancer Drugs. 2016. PMID: 27857884 Free PMC article.
-
Recombinant human lactoferrin induces apoptosis, disruption of F-actin structure and cell cycle arrest with selective cytotoxicity on human triple negative breast cancer cells.Apoptosis. 2019 Aug;24(7-8):562-577. doi: 10.1007/s10495-019-01539-7. Apoptosis. 2019. PMID: 30941553 Free PMC article.
-
Centchroman induces G0/G1 arrest and caspase-dependent apoptosis involving mitochondrial membrane depolarization in MCF-7 and MDA MB-231 human breast cancer cells.Life Sci. 2008 Mar 12;82(11-12):577-90. doi: 10.1016/j.lfs.2007.11.028. Epub 2007 Dec 15. Life Sci. 2008. PMID: 18279897
-
[Curcumin in chemoprevention of breast cancer].Postepy Hig Med Dosw (Online). 2014 Jan 2;68:571-8. doi: 10.5604/17322693.1102294. Postepy Hig Med Dosw (Online). 2014. PMID: 24864107 Review. Polish.
-
1,5-diaryl-3-oxo-1,4-pentadienes: a case for antineoplastics with multiple targets.Curr Med Chem. 2009;16(16):2001-20. doi: 10.2174/092986709788682218. Curr Med Chem. 2009. PMID: 19519378 Free PMC article. Review.
Cited by
-
A novel class of piperidones exhibit potent, selective and pro-apoptotic anti-leukemia properties.Oncol Lett. 2016 Jun;11(6):3842-3848. doi: 10.3892/ol.2016.4480. Epub 2016 Apr 20. Oncol Lett. 2016. PMID: 27313705 Free PMC article.
-
Tumor-selective cytotoxicity of a novel pentadiene analogue on human leukemia/ lymphoma cells.Clin Cancer Drugs. 2016;3(2):138-146. doi: 10.2174/2212697X03666160830165250. Clin Cancer Drugs. 2016. PMID: 27857884 Free PMC article.
-
Cytological Assessments and Transcriptome Profiling Demonstrate that Evodiamine Inhibits Growth and Induces Apoptosis in a Renal Carcinoma Cell Line.Sci Rep. 2017 Oct 3;7(1):12572. doi: 10.1038/s41598-017-12918-y. Sci Rep. 2017. PMID: 28974748 Free PMC article.
-
A Novel Pyrazole Exhibits Potent Anticancer Cytotoxicity via Apoptosis, Cell Cycle Arrest, and the Inhibition of Tubulin Polymerization in Triple-Negative Breast Cancer Cells.Cells. 2024 Jul 20;13(14):1225. doi: 10.3390/cells13141225. Cells. 2024. PMID: 39056806 Free PMC article.
-
Ni-Cu Nanoparticles and Their Feasibility for Magnetic Hyperthermia.Nanomaterials (Basel). 2020 Oct 9;10(10):1988. doi: 10.3390/nano10101988. Nanomaterials (Basel). 2020. PMID: 33050215 Free PMC article.
References
-
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. SEER Cancer Statistics Review, 1975-2012, National Cancer Institute. Bethesda, MD: http://seer.cancer.gov/csr/1975_2012/, based on November 2014 SEER data submission, posted to the SEER web site, April 2015. Accessed Jan 2016.
-
- Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J, Cristofanilli M, Dontu G, Bidaut L, Valero V, Hortobagyi GN, Yu D. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69:4951–4953. - PubMed
-
- Berardi DE, Flumian C, Campodonico PB, Urtreger AJ, Diaz Bessone MI, Motter AN, Bal de Kier Joffe ED, Farias EF, Todaro LB. Myoepithelial and luminal breast cancer cells exhibit different responses to all-trans retinoic acid. Cell. Oncol. 2015;38:289–305. doi:10.1007/s13402-015-0230-z. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

