A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: study protocol for a randomized controlled trial
- PMID: 26920137
- PMCID: PMC4768412
- DOI: 10.1186/s13063-016-1239-y
A prospective multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART Disc plus autologous disc chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disc to avoid secondary disease: study protocol for a randomized controlled trial
Abstract
Background: Intervertebral disc degeneration is emphasized as an important cause of low back pain. Current surgical treatment provides relief to the accompanying pain and disability but does not restore the biological function of the intervertebral disc. NOVOCART™ Disc plus, an autologous cell compound for autologous disc chondrocyte transplantation, was developed to reduce the degenerative sequelae after lumbar disc surgery or to prophylactically avoid degeneration in adjacent discs.
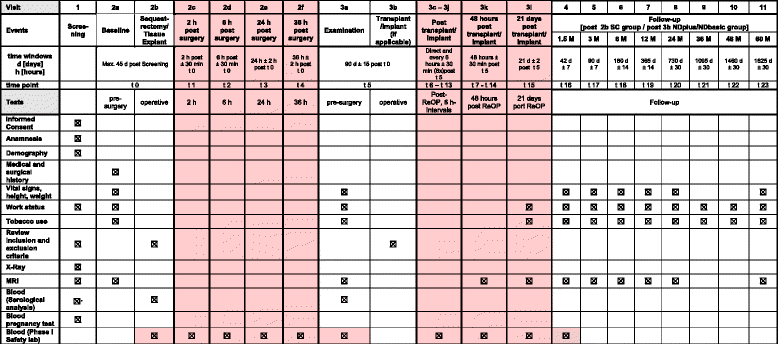
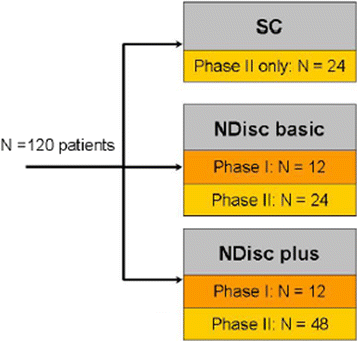
Methods/design: This is a multicenter, randomized, controlled, clinical phase I/II combination study. A total of 120 adult patients are allocated in a ratio of 2:1:1. Sample size and power calculations were performed to detect the minimal clinically important difference of 10 units, with an expected standard deviation of 12 in the Oswestry Disability Index, which is the primary outcome parameter. Secondary outcome parameters include the visual analog scale and the EQ-5D questionnaire. Changes in physical and mental health are evaluated using the Short Form-12 (SF-12). Moreover, radiological and functional outcomes are evaluated. The major inclusion criterion is a single lumbar disc herniation that requires sequestrectomy. Transplantation is performed 90 days thereafter. Study data generation (study sites) and data storage, processing, and statistical analysis are clearly separated.
Discussion: In this phase-I/II study, NDplus is being investigated for its clinical applicability, safety, and efficacy in the repair of herniated, nucleotomized discs, and of adjacent degenerated discs, if present. To date, autologous disc chondrocytes have not been transplanted into degenerative discs without previous disc herniation. As such, this is the first study to investigate a therapeutic as well as a prophylactic approach to treat degenerative discs of the lumbar spine.
Trial registration: EudraCT No: 2010-023830-22, ID NCT01640457 , 8 November 2010.
Figures
References
-
- Siddiqui AH, Rafique MZ, Ahmad MN, Usman MU. Role of magnetic resonance imaging in lumbar spondylosis. J Coll Physicians Surg Pak. 2005;15:396–9. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

