Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease
- PMID: 26920146
- PMCID: PMC4768417
- DOI: 10.1186/s40659-016-0073-8
Carotid body chemoreceptors, sympathetic neural activation, and cardiometabolic disease
Abstract
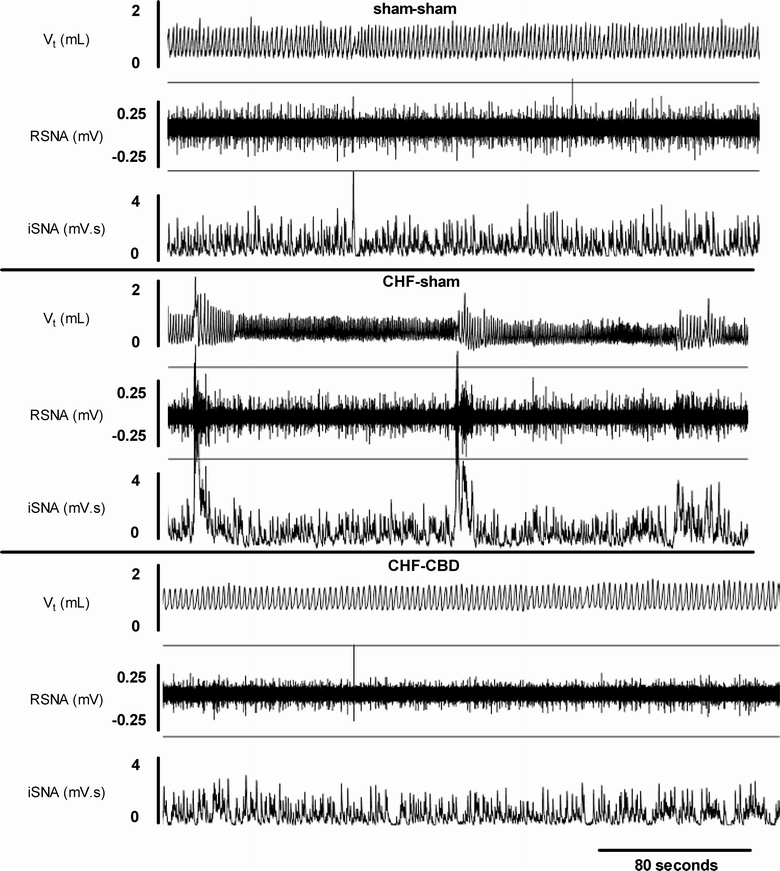
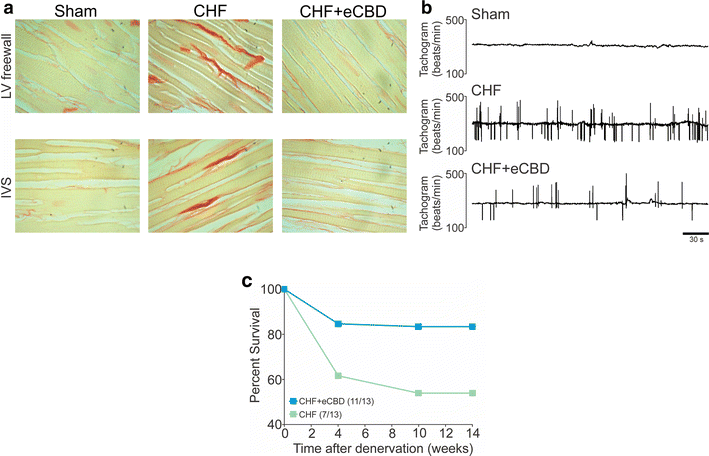
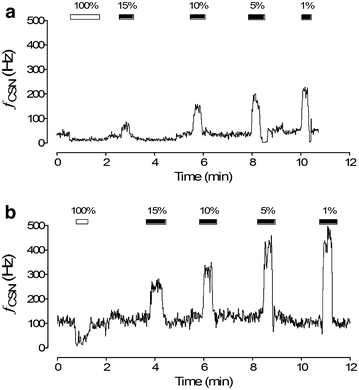
The carotid body (CB) is the main peripheral chemoreceptor that senses the arterial PO2, PCO2 and pH. In response to hypoxemia, hypercapnia and acidosis, carotid chemosensory discharge elicits reflex respiratory, autonomic and cardiovascular adjustments. The classical construct considers the CB as the main peripheral oxygen sensor, triggering reflex physiological responses to acute hypoxemia and facilitating the ventilatory acclimation to chronic hypoxemia at high altitude. However, a growing body of experimental evidence supports the novel concept that an abnormally enhanced CB chemosensory input to the brainstem contributes to overactivation of the sympathetic nervous system, and consequent pathology. Indeed, the CB has been implicated in several diseases associated with increases in central sympathetic outflow. These include hypertension, heart failure, sleep apnea, chronic obstructive pulmonary disease and metabolic syndrome. Indeed, ablation of the CB has been proposed for the treatment of severe and resistant hypertension in humans. In this review, we will analyze and discuss new evidence supporting an important role for the CB chemoreceptor in the progression of autonomic and cardiorespiratory alterations induced by heart failure, obstructive sleep apnea, chronic obstructive pulmonary disease and metabolic syndrome.
Figures
References
-
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

