Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation
- PMID: 26920550
- PMCID: PMC4768407
- DOI: 10.1186/s12974-016-0512-z
Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation
Abstract
Background: Conditions of inflammatory tissue distress are associated with high extracellular levels of adenosine, due to increased adenosine triphosphate (ATP) degradation upon cellular stress or the release of extracellular ATP upon cell death, which can be degraded to adenosine by membrane-bound ecto-enzymes like CD39 and CD73. Adenosine is recognised to mediate anti-inflammatory effects via the adenosine A2a receptor (A2aR), as shown in experimental models of arthritis. Here, using pharmacological interventions and genetic inactivation, we investigated the roles of A2aR in experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS).
Methods: We used two independent mouse EAE variants, i.e. active immunization in C57BL/6 with myelin oligodendrocyte glycoprotein (MOG)35-55 or transfer-EAE by proteolipid protein (PLP)139-155-stimulated T lymphocytes and EAE in mice treated with A2aR-agonist CGS21680 at different stages of disease course and in mice lacking A2aR (A2aR(-/-)) compared to direct wild-type littermates. In EAE, we analysed myelin-specific proliferation and cytokine synthesis ex vivo, as well as inflammation and demyelination by immunohistochemistry. In vitro, we investigated the effect of A2aR on migration of CD4(+) T cells, macrophages and microglia, as well as the impact of A2aR on phagocytosis of macrophages and microglia. Statistical tests were Mann-Whitney U and Student's t test.
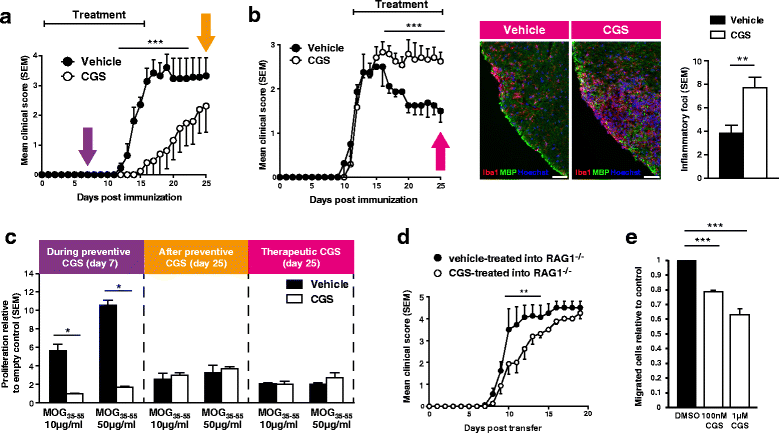
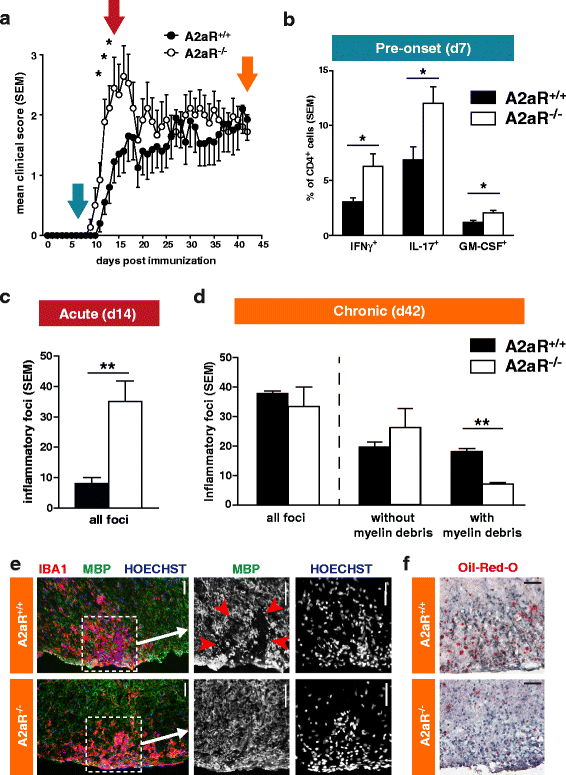
Results: We found an upregulation of A2aR in the central nervous system (CNS) in EAE, predominantly detected on T cells and macrophages/microglia within the inflamed tissue. Preventive EAE treatment with A2aR-specific agonist inhibited myelin-specific T cell proliferation ex vivo and ameliorated disease, while application of the same agonist after disease onset exacerbated non-remitting EAE progression and resulted in more severe tissue destruction. Accordingly, A2aR-deficient mice showed accelerated and exacerbated disease manifestation with increased frequencies of IFN-γ-, IL-17- and GM-CSF-producing CD4(+) T helper cells and higher numbers of inflammatory lesions in the early stage. However, EAE quickly ameliorated and myelin debris accumulation was lower in A2aR(-/-) mice. In vitro, activation of A2aR inhibited phagocytosis of myelin by macrophages and primary microglia as well as migration of CD4(+) T cells, macrophages and primary microglia.
Conclusions: A2aR activation exerts a complex pattern in chronic autoimmune neurodegeneration: while providing anti-inflammatory effects on T cells and thus protection at early stages, A2aR seems to play a detrimental role during later stages of disease and may thus contribute to sustained tissue damage within the inflamed CNS.
Figures


References
-
- Blackburn MR, Vance CO, Morschl E, Wilson CN. Adenosine receptors and inflammation. Handb Exp Pharmacol. 2009;215–269. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

